Relative Efficiency of Pitfall vs. Bait Trapping for Capturing Taxonomic and Functional Diversities of Ant Assemblages in Temperate Heathlands
Abstract
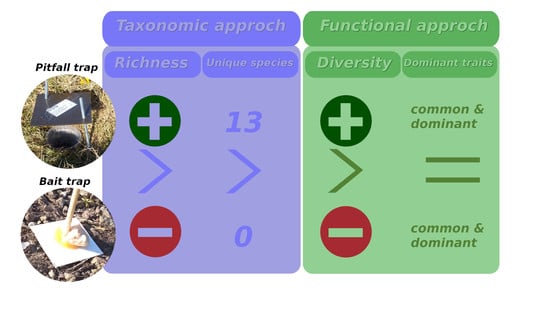
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Sampling Design
2.3. Functional Traits
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Headcount | Size | Dominance | Trophic Guild | Dispersal | Colony Size Score | Foraging Distance (in Meter) | Stratum | |
|---|---|---|---|---|---|---|---|---|---|
| Pitfall | Bait | ||||||||
| Aphaenogaster gibbosa (Latreille, 1798) | 21 | 0 | L | 0 | Om | I | 6.48 | 2 | E |
| Aphaenogaster subterranea (Latreille, 1798) | 4 | 0 | M | 0 | Om | I | 7.6 | 2 | E |
| Formica cunicularia Latreille, 1798 | 93 | 42 | L | 0 | Om | I | 7.24 | 20 | E |
| Formica pratensis Retzius, 1783 | 1960 | 75 | L | 1 | Om | I | 11 | 100 | E |
| Hypoponera eduardi (Forel, 1894) | 6 | 0 | S | 0 | Pr | M | 7.31 | 2 | H |
| Lasius alienus (Foerster, 1850) | 110 | 778 | M | 1 | Om | I | 9.47 | 10 | E |
| Lasius emarginatus (Olivier, 1792) | 1 | 0 | M | 1 | Om | I | 9.21 | 10 | E |
| Lasius flavus (Fabricius, 1782) | 8 | 0 | M | 0 | Ne | I | 9.21 | 2 | H |
| Lasius niger (Linnaeus, 1758) | 962 | 498 | M | 1 | Ne | I | 9.21 | 10 | E |
| Lasius platythorax (Seifert, 1991) | 30 | 17 | M | 1 | Ne | I | 9.21 | 5 | E |
| Lasius psammophilus (Seifert, 1992) | 66 | 36 | M | 0 | Ne | I | 10.43 | 5 | E |
| Messor capitatus (Latreille, 1798) | 7 | 0 | L | 1 | SF | I | 8.29 | 20 | E |
| Myrmecina graminicola (Latreille, 1802) | 5 | 0 | M | 0 | Pr | I | 4.61 | 2 | H |
| Myrmica ruginodis Nylander, 1846 | 29 | 0 | L | 0 | Om | M | 7.6 | 2 | E |
| Myrmica sabuleti Meinert, 1860 | 45 | 44 | L | 0 | Om | M | 8.01 | 2 | E |
| Myrmica scabrinodis Nylander, 1846 | 530 | 50 | L | 0 | Om | M | 7.31 | 2 | E |
| Plagiolepis pallescens Forel, 1889 | 8 | 0 | S | 0 | Om | M | 6.68 | 5 | E |
| Ponera coarctata (Latreille, 1802) | 1 | 0 | M | 0 | Pr | D | 4.61 | 2 | H |
| Solenopsis fugax (Latreille, 1798) | 2 | 0 | S | NA | Om | M | NA | 2 | H |
| Tapinoma erraticum (Latreille, 1798) | 176 | 88 | S | 1 | Om | M | 8.16 | 10 | E |
| Temnothorax unifasciatus (Latreille, 1798) | 1 | 0 | S | 0 | Pr | I | 5.78 | 2 | E |
| Tetramorium atratulum (Schenk, 1852) | 1 | 0 | S | 0 | Pa | P | 0 | NA | NA |
| Tetramorium gr. caespitum-impurum | 910 | 2791 | S | 1 | Om | I | 9.21 | 10 | E |
Appendix B
| Trait | Data Type | States | References |
|---|---|---|---|
| Size | Categorical | Worker body size from the tip of mandibles to tip of the gaster (mm): Small: <3 mm Medium: 3–4 mm Large: >4 mm | [13] [30] |
| Dominance | Binary | 0: Subordinate | [50] |
| 1: Dominant | |||
| Trophic guild | Categorical | Omnivore | [50] |
| Predator | [30] [51] | ||
| Seed feeder | |||
| Nectarivorous | |||
| Parasitic | |||
| Dispersal | Categorical | Independent | [50] |
| Dependent | [30] | ||
| Mixed | [51] | ||
| Parasitic | |||
| Colony size | Numerical | Logarithm of mean of number of workers per colony | [50] |
| Foraging distance | Numerical | Distance in meters from the nest while foraging | [52] [53] [54] [55] [56] [57] [58] [59] |
| Stratum | Categorical | Epigaeic Hypogaeic | [55] [59] |
References
- Lach, L.; Parr, C.; Abbott, K. (Eds.) Ant Ecology; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Andersen, A.N.; Majer, J.D. Ants show the way Down Under: Invertebrates as bioindicators in land management. Front. Ecol. Environ. 2004, 2, 291–298. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellison, A.M.; Record, S.; Arguello, A.; Gotelli, N.J. Rapid inventory of the ant assemblage in a temperate hardwood forest: Species composition and assessment of sampling methods. Environ. Entomol. 2007, 36, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Gerlash, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Higgins, R.J.; Lindgren, B.S. An evaluation of methods for sampling ants (Hymenoptera: Formicidae) in British Columbia. Canada. Can. Entomol. 2012, 144, 491–507. [Google Scholar] [CrossRef]
- King, J.R.; Porter, S.D. Evaluation of sampling methods and species richness estimators for ants in upland ecosystems in Florida. Environ. Entomol. 2005, 34, 1566–1578. [Google Scholar] [CrossRef] [Green Version]
- Romero, H.; Jaffe, K. A comparison of methods for sampling ants (Hymenoptera: Formicidae) in Savannas. Biotropica 2014, 21, 348–352. [Google Scholar] [CrossRef] [Green Version]
- De Souza, J.L.P.; Baccaro, F.B.; Landeiro, V.L.; Franklin, E.; Magnusson, W.E. Trade-offs between complementarity and redundancy in the use of different sampling techniques for ground-dwelling ant assemblages. Appl. Soil Ecol. 2012, 56, 63–73. [Google Scholar] [CrossRef]
- Lang, A. The pitfalls of pitfalls: A comparison of pitfalls trap catches and absolute density estimates of epigeal invertebrate predators in arable land. J. Pest. Sci. 2000, 73, 99–106. [Google Scholar]
- Majer, J.D. The use of pitfall traps for sampling ants—A critique. Mem. Mus. Vic. 1997, 56, 323–329. [Google Scholar] [CrossRef]
- Sheikh, A.H.; Ganaie, G.A.; Thomas, M.; Bhandari, R.; Rather, Y.A. Ant pitfall trap sampling: An overview. J. Entomol. Res. 2018, 42, 421–436. [Google Scholar] [CrossRef]
- Agosti, D.; Majer, J.; Alonso, L.E.; Schultz, T. Ants: Standard Methods for Measuring and Monitoring Biodiversity; Smithsonian Institution Press: Wachington, DC, USA; London, UK, 2000. [Google Scholar]
- Ward, D.F.; New, T.R.; Yen, A.L. Effects of pitfall trap spacing on the abundance, richness and composition of invertebrate catches. J. Insect Conserv. 2001, 5, 47–53. [Google Scholar] [CrossRef]
- Lopes, C.T.; Vasconcelos, H.L. Evaluation of three methods for sampling ground-dwelling ants in the Brazilian Cerrado. Neotrop. Entomol. 2008, 37, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Adis, J. Problems for interpreting arthropod sampling with pitfall traps. Zool. Anziger Jena 1979, 202, 177–184. [Google Scholar]
- Luff, M.L. Some features influencing the efficiency of pitfall traps. Oecologia 1975, 19, 345–357. [Google Scholar] [CrossRef]
- Steiner, F.M.; Schlick-Steiner, B.C.; Moder, K.; Bruckner, A.; Christian, E. Congruence of data from different trapping periods of ant pitfall catches (Hymenoptera: Formicidae). Sociobiology 2005, 46, 105–116. [Google Scholar]
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Ellison, A.M. Out of Oz: Opportunities and challenges for using ants (Hymenoptera: Formicidae) as biological indicators in north-temperate cold biomes. Myrmecol. News. 2012, 17, 105–119. [Google Scholar]
- Lessard, J.P.; Dunn, R.R.; Sanders, N.J. Temperature-mediated coexistence in temperate forest ant communities. Insectes Soc. 2009, 56, 149–156. [Google Scholar] [CrossRef]
- Véle, A.; Holuša, J.; Frouz, J. Sampling for ants in different-aged spruce forests: A comparison of methods. Eur. J. Soil Biol. 2009, 45, 301–305. [Google Scholar] [CrossRef]
- Wang, C.; Strazanac, J.; Butler, L. A comparison of pitfall traps with bait traps for studying leaf litter ant communities. J. Econ. Entomol. 2001, 94, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Privet, K.; Pétillon, J. Comparative patterns in taxonomic and functional spider diversities between tropical vs. temperate forests. Ecol. Evol. 2020, 10, 13165–13172. [Google Scholar] [CrossRef] [PubMed]
- Kaspari, M.; Ward, P.S.; Yuan, M. Energy gradients and the geographic distribution of local ant diversity. Oecologia 2004, 140, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Bioret, F.; Bouzillé, J.B.; de Foucault, B.; Géhu, J.M.; Godeau, M. Le système thermo-atlantique pelouses-landes-fourrés des falaises des îles sud-armoricaines (Groix, Belle-Ile, Yeu). Doc. Phytosociol. 1988, 11, 513–531. [Google Scholar]
- Hacala, A.; Leroy, M.; Sawtschuk, J.; Pétillon, J. Comparative responses of spiders and plants to maritime heathland restoration. Biodivers. Conserv. 2020, 29, 229–249. [Google Scholar] [CrossRef]
- Association Infoclimat. Infoclimat-la Météo en Temps Réel. Available online: https://www.infoclimat.fr/ (accessed on 16 January 2021).
- Topping, C.J.; Sunderland, K.D. Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. J. Appl. Ecol. 1992, 29, 485–491. [Google Scholar] [CrossRef]
- Blatrix, R.; Galkowski, C.; Lebas, C.; Wegnez, P. Guide des Fourmis de France; Delachaux et Niestlé: Paris, France, 2013. [Google Scholar]
- Seifert, B.; Schultz, R. A taxonomic revision of the Formica rufibarbis FABRICIUS, 1793 group (Hymenoptera: Formicidae). Myrmecol. News. 2009, 12, 255–272. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlags-und Vertriebsgesellschaft: Tauer, Germany, 2018. [Google Scholar]
- Seifert, B. A taxonomic revision of the Palaearctic members of the subgenus Lasius s.str. (Hymenoptera, Formicidae). Soil Org. 2020, 92, 15–86. [Google Scholar] [CrossRef]
- Menke, S.B.; Vachter, N. A comparison of the effectiveness of pitfall traps and winkler litter samples for characterization of terrestrial ant (Formicidae) communities in temperate savannas. Great Lakes Entomol. 2018, 47, 4. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Commun. Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. Package ‘FD’. Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. 2015. Available online: https://mran.microsoft.com/snapshot/2014-11-17/web/packages/FD/FD.pdf (accessed on 1 November 2019).
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. Program and User’s Guide. 2016. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/ (accessed on 1 November 2019).
- Majer, J.D.; Beeston, G. The Biodiversity Integrity Index: An Illustration Using Ants in Western Australia. Conserv. Biol. 1996, 10, 65–73. [Google Scholar] [CrossRef] [Green Version]
- O’hara, R.B.; Kotze, D.J. Do not log-transform count data. Methods Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef]
- Fourcassié, V.J.; Schatz, B.; Beugnon, G. Temporal information in social insects. In Information Processing in Social Insects; Birkhäuser: Basel, Switzerland, 1999; pp. 261–275. [Google Scholar]
- Tavares, A.A.; Bispo, P.C.; Zanzini, A.C. Effect of collect time on communities of epigaeic ants (Hymenoptera: Formicidae) in areas of Eucalyptus cloeziana and cerrado. Neotrop. Entomol. 2008, 37, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Gotelli, N.J.; Ellison, A.M.; Dunn, R.R.; Sanders, N.J. Counting ants (Hymenoptera: Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecol. News. 2011, 15, 13–19. [Google Scholar]
- Boet, O.; Arnan, X.; Retana, J. The role of environmental vs. biotic filtering in the structure of European ant communities: A matter of trait type and spatial scale. PLoS ONE 2020, 15, e0228625. [Google Scholar] [CrossRef] [Green Version]
- Witté, I.; Touroult, J. Répartition de la biodiversité en France métropolitaine: Une synthèse des Atlas faunistiques. VertigO La Revue Électronique en Sciences de L’environnement 2014, 14. Available online: http://journals.openedition.org/vertigo/14645 (accessed on 16 March 2021). [CrossRef]
- Mahon, M.B.; Campbell, K.U.; Crist, T.O. Effectiveness of Winkler litter extraction and pitfall traps in sampling ant communities and functional groups in a temperate forest. Environ. Entomol. 2017, 46, 470–479. [Google Scholar] [CrossRef]
- Arnan, X.; Cerdá, X.; Retana, J. Partitioning the impact of environment and spatial structure on alpha and beta components of taxonomic, functional, and phylogenetic diversity in European ants. PeerJ 2015, 3, e1241. [Google Scholar] [CrossRef] [Green Version]
- Dekoninck, W.; Royal Belgian Institute of Natural Sciences, Brussels, Belgium. Personal Communication, 2020.
- Domisch, T.; Neuvonen, S.; Sundström, L.; Punttila, P.; Finér, L.; Kilpeläinen, J.; Niemelä, P.; Risch, A.C.; Ohashi, M.; Jurgensen, M.F. Sources of variation in the incidence of ant–aphid mutualism in boreal forests. Agric. For. Entomol. 2011, 13, 239–245. [Google Scholar] [CrossRef]
- Epps, M.J.; Penick, C.A. Facultative mushroom feeding by common woodland ants (Formicidae, Aphaenogaster spp.). Food Webs 2017, 14, 9–13. [Google Scholar] [CrossRef]
- Fokuhl, G.; Heinze, J.; Poschlod, P. Myrmecochory by small ants–Beneficial effects through elaiosome nutrition and seed dispersal. Acta Oecol. 2012, 38, 71–76. [Google Scholar] [CrossRef]
- Gouraud, C.; University of Rennes1, Rennes, France. Personal Communication, 2020.
- Lázaro-González, A.; Arnan, X.; Boulay, R.; Cerdá, X.; Rodrigo, A. Short-term ecological and behavioural responses of Mediterranean ant species Aphaenogaster gibbosa (L atr. 1798) to wildfire. Insect Conserv. Divers. 2013, 6, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Plowes, N.J.; Johnson, R.A.; Hoelldobler, B. Foraging behavior in the ant genus Messor (Hymenoptera: Formicidae: Myrmicinae). Myrmecol. News. 2013, 18, 33–49. [Google Scholar]
- Puissauve, R. Gestion d’une Population de M. arion sur l’Éperon Murat—État des Lieux. Objectif: Évaluer la Population de M. arion et de ses Différents hôTes (Origan et Myrmica sabuleti) en vue de Lancer un Plan de Gestion Conservatoire sur le Site (Université François Rabelais de Tours, Conservatoire du Patrimoine Naturel de la région Centre, UMR-CNRS 6035 Sociobiologie des Fourmis); University of Tours: Tours, France, 2007. [Google Scholar]
- Schlick-Steiner, B.C.; Steiner, F.M.; Moder, K.; Bruckner, A.; Fiedler, K.; Christian, E. Assessing ant assemblages: Pitfall trapping versus nest counting (Hymenoptera, Formicidae). Insectes Soc. 2006, 53, 274–281. [Google Scholar] [CrossRef]





| Species | S1 | S2 | S3 | All Sites | ||||
|---|---|---|---|---|---|---|---|---|
| Bait | Pitfall | Bait | Pitfall | Bait | Pitfall | Bait | Pitfall | |
| Aphaenogaster gibbosa (Latreille, 1798) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Aphaenogaster subterranea (Latreille, 1798) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Formica cunicularia Latreille, 1798 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Formica pratensis Retzius, 1783 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| Hypoponera eduardi (Forel, 1894) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 |
| Lasius alienus (Foerster, 1850) | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Lasius emarginatus (Olivier, 1792) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Lasius flavus (Fabricius, 1782) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Lasius niger (Linnaeus, 1758) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Lasius platythorax Seifert, 1991 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Lasius psammophilus Seifert, 1992 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| Messor capitatus (Latreille, 1798) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Myrmecina graminicola (Latreille, 1802) | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Myrmica ruginodis Nylander, 1846 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Myrmica sabuleti Meinert, 1860 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Myrmica scabrinodis Nylander, 1846 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Plagiolepis pallescens Forel, 1894 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Ponera coarctata (Latreille, 1802) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Solenopsis fugax (Latreille, 1798) | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Tapinoma erraticum (Latreille, 1798) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Temnothorax unifasciatus (Latreille, 1798) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Tetramorium atratulum (Schenck, 1852) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Tetramorium gr. caespitum-impurum | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Only bait/shared/only pitfall |  |  |  |  | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacala, A.; Gouraud, C.; Dekoninck, W.; Pétillon, J. Relative Efficiency of Pitfall vs. Bait Trapping for Capturing Taxonomic and Functional Diversities of Ant Assemblages in Temperate Heathlands. Insects 2021, 12, 307. https://doi.org/10.3390/insects12040307
Hacala A, Gouraud C, Dekoninck W, Pétillon J. Relative Efficiency of Pitfall vs. Bait Trapping for Capturing Taxonomic and Functional Diversities of Ant Assemblages in Temperate Heathlands. Insects. 2021; 12(4):307. https://doi.org/10.3390/insects12040307
Chicago/Turabian StyleHacala, Axel, Clément Gouraud, Wouter Dekoninck, and Julien Pétillon. 2021. "Relative Efficiency of Pitfall vs. Bait Trapping for Capturing Taxonomic and Functional Diversities of Ant Assemblages in Temperate Heathlands" Insects 12, no. 4: 307. https://doi.org/10.3390/insects12040307