Optical and Thermal Investigations of Eutectic Metallomesogen Mixtures Based on Salicylaldiaminates Metal Complexes with a Large Nematic Stability Range
Abstract
:1. Introduction
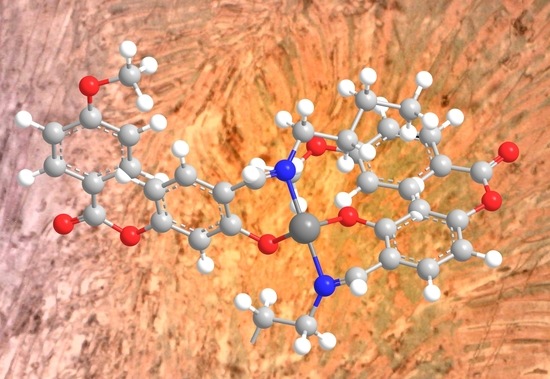
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of 12-8NCu
3.4. Synthesis of A6O-8NCu and A11O-6ONPd
3.5. Synthesis of A11O-6ONNi
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, H.; Yu, R.; Ma, Z.; Gong, Y.; Zhao, B.; Lv, Q.; Tan, Z.A. Recent advances of organometallic complexes in emerging photovoltaics. J. Polym. Sci. 2021, 60, 865–916. [Google Scholar] [CrossRef]
- Almosni, S.; Delamarre, A.; Jehl, Z.; Suchet, D.; Cojocaru, L.; Giteau, M.; Behaghel, B.; Julian, A.; Ibrahim, C.; Tatry, L.; et al. Material challenges for solar cells in the twenty-first century: Directions in emerging technologies. Sci. Technol. Adv. Mater. 2018, 19, 336–369. [Google Scholar] [CrossRef]
- Kadkin, O.N.; An, J.; Han, H.; Galyametdinov, Y.G. A Novel Series of Heteropolynuclear Metallomesogens: Organopalladium Complexes with Ferrocenophane-Containing Ligands. Eur. J. Inorg. Chem. 2008, 2008, 1682–1688. [Google Scholar] [CrossRef]
- Wang, Y.; Cabry, C.P.; Xiao, M.; Male, L.; Cowling, S.J.; Bruce, D.W.; Shi, J.; Zhu, W.; Baranoff, E. Blue and Green Phosphorescent Liquid-Crystalline Iridium Complexes with High Hole Mobility. Chem. Eur. J. 2016, 22, 1618–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippard, S.J. Platinum complexes: Probes of polynucleotide structure and antitumor drugs. Acc. Chem. Res. 2002, 11, 211–217. [Google Scholar] [CrossRef]
- Jennette, K.W.; Lippard, S.J.; Vassiliades, G.A.; Bauer, W.R. Metallointercalation reagents. 2-hydroxyethanethiolato(2,2′,2′-terpyridine)-platinum(II) monocation binds strongly to DNA by intercalation. Proc. Natl. Acad. Sci. USA 1974, 71, 3839–3843. [Google Scholar] [CrossRef] [Green Version]
- Sethupathi, M.; Thulasinathan, B.; Sengottuvelan, N.; Ponnuchamy, K.; Perdih, F.; Alagarsamy, A.; Karthikeyan, M. Macrocyclic “tet a”-Derived Cobalt(III) Complex with a N,N′-Disubstituted Hexadentate Ligand: Crystal Structure, Photonuclease Activity, and as a Photosensitizer. ACS Omega 2022, 7, 669–682. [Google Scholar] [CrossRef]
- Mocerino, F.; Pezzella, A.; Caruso, U. Eumelanin pigment precursor 2-carboxy-5,6-dihydroxyindole and 2-amino-6-methylbenzothiazole chromophore integration towards melanin inspired chemoresponsive materials: The case of the Zn(2+) ion. RSC Adv. 2022, 12, 21050–21055. [Google Scholar] [CrossRef]
- Katari, M.; Payen de la Garanderie, E.; Nicol, E.; Steinmetz, V.; van der Rest, G.; Carmichael, D.; Frison, G. Combining gas phase electron capture and IRMPD action spectroscopy to probe the electronic structure of a metastable reduced organometallic complex containing a non-innocent ligand. Phys. Chem. Chem. Phys. 2015, 17, 25689–25692. [Google Scholar] [CrossRef] [Green Version]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling buildingblock. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Liao, T.C.; Li, Z.Q.; Gonzalez-Garcia, J.; Reynolds, M.; Zou, M.; Vilar, R. Dinickel-Salphen Complexes as Binders of Human Telomeric Dimeric G-Quadruplexes. Chemistry 2017, 23, 4713–4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaphan, D.M.; Klet, R.C.; Perras, F.A.; Pruski, M.; Yang, C.; Kropf, A.J.; Delferro, M. Surface Organometallic Chemistry of Supported Iridium(III) as a Probe for Organotransition Metal-Support Interactions in C-H Activation. ACS Catal. 2018, 8, 5363–5373. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-T.; Wang, Y.-J.; Huang, C.-S.; Sheu, H.-S.; Lee, G.-H.; Lai, C.K. New metallomesogens derived from unsymmetric 1,3,4-thiodiazoles: Synthesis, single crystal structure, mesomorphism, and optical properties. Tetrahedron 2007, 63, 12437–12445. [Google Scholar] [CrossRef]
- Shenderovich, I.G. 1,3,5-triaza-7-phosphaadamantane (PTA) as a 31P NMR probe for organometallic transition metal complexes in solution. Molecules 2021, 26, 1390. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, P.; Gao, F.; Zhang, S.; Li, X. A displacement-type fluorescent probe reveals active species in the coordinative polymerization of olefins. Polym. Chem. 2018, 9, 603–610. [Google Scholar] [CrossRef]
- Teets, T.S.; Wu, Y. Organometallic Photosensitizers. In Comprehensive Organometallic Chemistry IV; Parkin, G., Meyer, K., O’hare, D., Eds.; Elsevier: Oxford, UK, 2022; pp. 284–338. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. A symmetrical azo-based fluorophore and the derived salen multipurpose framework for emissive layers. Inorg. Chem. Commun. 2019, 104, 186–189. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Tuzi, A.; Caruso, U. Two tridentate pyridinyl-hydrazone zinc(II) complexes as fluorophores for blue emitting layers. J. Mol. Struct. 2019, 1197, 672–680. [Google Scholar] [CrossRef]
- Easter, Q.T.; Blum, S.A. Organic and Organometallic Chemistry at the Single-Molecule, -Particle, and -Molecular-Catalyst-Turnover Level by Fluorescence Microscopy. Acc. Chem. Res. 2019, 52, 2244–2255. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Peng, X.; Yoon, J. Fluorescent and colorimetric probes for detection of thiols. Chem. Soc. Rev. 2010, 39, 2120–2135. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Tuzi, A.; Piotto, S.; Concilio, S.; Caruso, U. An amphiphilic pyridinoyl-hydrazone probe for colorimetric and fluorescence pH sensing. Molecules 2019, 24, 3833. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Chen, P.; Zong, L.; Sun, Y.; Liu, G.; Yu, X.; Qin, J. Colorimetric and fluorescent probes for real-time naked eye sensing of copper ion in solution and on paper substrate. R Soc. Open Sci. 2017, 4, 171161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diana, R.; Caruso, U.; Di Costanzo, L.; Gentile, F.S.; Panunzi, B. Colorimetric recognition of multiple first-row transition metals: A single water-soluble chemosensor in acidic and basic conditions. Dye Pigm. 2021, 184, 108832. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Tuzi, A.; Panunzi, B. A highly water-soluble fluorescent and colorimetric pH probe. Crystals 2020, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Song, R.; Feng, H.; Guo, K.; Meng, Q.; Chi, H.; Zhang, R.; Zhang, Z. Visualization of Fluoride Ions In Vivo Using a Gadolinium(III)-Coumarin Complex-Based Fluorescence/MRI Dual-Modal Probe. Sensors 2016, 16, 2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diana, R.; Caruso, U.; Concilio, S.; Piotto, S.; Tuzi, A.; Panunzi, B. A real-time tripodal colorimetric/fluorescence sensor for multiple target metal ions. Dye Pigm. 2018, 155, 249–257. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, D.; Li, H.; Li, Q.; Yang, Y. A Colorimetric Probe for Determination of Trace Pb(II) in Tea Based on Peroxidase-like Activity of Carbon Dots Co-doped with Copper and Iodine. Shipin Kexue 2021, 42, 332–337. [Google Scholar]
- Laosuwan, M.; Poonsawat, C.; Burakham, R.; Srijaranai, S.; Mukdasai, S. A novel liquid colorimetric probe for highly selective and sensitive detection of lead (II). Food Chem. 2021, 363, 130254. [Google Scholar] [CrossRef]
- Ma, X.; Liu, L.; Wang, J.; Hao, Y.; Xu, X.; Shang, X. The Role of Hydrazine in Colorimetric Probes Based on Ferrocene Derivative. Helv. Chim. Acta 2022, 105, e202200037. [Google Scholar] [CrossRef]
- Qin, A.; Zhang, Y.; Gong, S.; Li, M.; Gao, Y.; Xu, X.; Song, J.; Wang, Z.; Wang, S. A novel flavonol-based colorimetric and turn-on fluorescent probe for rapid determination of hydrazine in real water samples and its bioimaging in vivo and in vitro. Front. Chem. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, C.; Yang, M.; Wen, S.; Zhang, J. Characterization of a Hg2+-selective fluorescent probe based on rhodamine B and its imaging in living cells. Molecules 2021, 26, 3385. [Google Scholar] [CrossRef]
- Cummings, S.D. Platinum complexes of terpyridine: Interaction and reactivity with biomolecules. Coord. Chem. Rev. 2009, 253, 1495–1516. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, M.; Taghizadeh, A.; Vahabi, H.; Habibzadeh, S.; Formela, K.; Saeb, M.R. Metal-Organic Framework (MOF)/Epoxy Coatings: A Review. Materials 2020, 13, 2881. [Google Scholar] [CrossRef] [PubMed]
- Benali, A.; Luo, Y.; Shin, H.; Pahls, D.; Heinonen, O. Quantum Monte Carlo Calculations of Catalytic Energy Barriers in a Metallorganic Framework with Transition-Metal-Functionalized Nodes. J. Phys. Chem. C 2018, 122, 16683–16691. [Google Scholar] [CrossRef]
- Chavan, S.; Bonino, F.; Vitillo, J.G.; Groppo, E.; Lamberti, C.; Dietzel, P.D.C.; Zecchina, A.; Bordiga, S. Response of CPO-27-Ni towards CO, N2 and C2H4. Phys. Chem. Chem. Phys. PCCP 2009, 11, 9811–9822. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, M.; Liu, S.; Swager, T.M. Columnar liquid crystallinity and mechanochromism in cationic platinum(II) complexes. J. Am. Chem. Soc. 2014, 136, 2952–2955. [Google Scholar] [CrossRef] [Green Version]
- Geng, H.; Luo, K.; Cheng, H.; Zhang, S.; Ni, H.; Wang, H.; Yu, W.; Li, Q. Novel columnar metallomesogens based on cationic platinum(ii) complexes without long peripheral chains. RSC Adv. 2017, 7, 11389–11393. [Google Scholar] [CrossRef] [Green Version]
- Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The effect of bulky substituents on two π-conjugated mesogenic fluorophores. Their organic polymers and zinc-bridged luminescent networks. Polymers 2019, 11, 1379. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fan, J.; Shi, J.; Qi, H.; Baranoff, E.; Xie, G.; Li, Q.; Tan, H.; Liu, Y.; Zhu, W. Influence of integrated alkyl-chain length on the mesogenic and photophysical properties of platinum-based metallomesogens and their application for polarized white OLEDs. Dye Pigm. 2016, 133, 238–247. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, M.-S.; Chen, L.-Y.; Hong, Y.-H.; Tsai, C.-H.; Wu, C.-C.; Poloek, A.; Chi, Y.; Chen, C.-A.; Chen, S.H. Polarized phosphorescent organic light-emitting devices adopting mesogenic host-guest systems. Org. Electron. Phys. Mater. Appl. 2011, 12, 15–21. [Google Scholar] [CrossRef]
- Caruso, U.; Centore, R.; Roviello, A.; Sirigu, A. Synthesis and Preliminary Characterization of a New Fully Aromatic Mesogenic Polyester Containing a 2-Phenylbenzoxazole Group. Macromolecules 1992, 25, 2290–2293. [Google Scholar] [CrossRef]
- Yang, X.; Wu, X.; Zhou, D.; Yu, J.; Xie, G.; Bruce, D.W.; Wang, Y. Platinum-based metallomesogens bearing a Pt(4,6-dfppy)(acac) skeleton: Synthesis, photophysical properties and polarised phosphorescence application. Dalton Trans. 2018, 47, 13368–13377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, A.J.; Martinho, P.N.; Gildea, B.J.; Holbrey, J.D.; Morgan, G.G. Robust Room Temperature Hysteresis in an FeIII Spin Crossover Metallomesogen. Eur. J. Inorg. Chem. 2015, 2016, 2025–2029. [Google Scholar] [CrossRef] [Green Version]
- Akiyoshi, R.; Zenno, H.; Sekine, Y.; Nakaya, M.; Akita, M.; Kosumi, D.; Lindoy, L.F.; Hayami, S. A Ferroelectric Metallomesogen Exhibiting Field-Induced Slow Magnetic Relaxation. Chem. Eur. J. 2022, 28, e202103367. [Google Scholar] [CrossRef]
- Binnemans, K.; Malykhina, L.; Mironov, V.S.; Haase, W.; Driesen, K.; Van Deun, R.; Fluyt, L.; Gorller-Walrand, C.; Galyametdinov, Y.G. Probing the magnetic anisotropy of lanthanide-containing metallomesogens by luminescence spectroscopy. ChemPhysChem 2001, 2, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Godbert, N.; Crispini, A.; Termine, R.; Golemme, A.; Ghedini, M. Photoconductive Nile red cyclopalladated metallomesogens. J. Mater. Chem. 2012, 22, 23617–23626. [Google Scholar] [CrossRef]
- Su, P.Y.; Tseng, J.C.; Lee, K.M.; Wang, J.C.; Lin, I.J. Tetranuclear silver(I) clusters showing high ionic conductivity in a bicontinuous cubic mesophase. Inorg. Chem. 2014, 53, 5902–5910. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Kang, D.-G.; Lee, M.-H.; Kim, J.-S.; Lee, C.-R.; Jeong, K.-U. A photo-responsive metallomesogen for an optically and electrically tunable polarized light modulator. Chem. Commun. 2016, 52, 12821–12824. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Lub, J.; Steenbakkers, J.A.M.; Broer, D.J. High-Contrast Thin-Film Polarizers by Photo-Crosslinking of Smectic Guest-Host Systems. Adv. Mater. 2006, 18, 2412–2417. [Google Scholar] [CrossRef]
- Gutierrez-Cuevas, K.G.; Wang, L.; Xue, C.; Singh, G.; Kumar, S.; Urbas, A.; Li, Q. Near infrared light-driven liquid crystal phase transition enabled by hydrophobic mesogen grafted plasmonic gold nanorods. Chem. Commun. 2015, 51, 9845–9848. [Google Scholar] [CrossRef]
- Wang, T.J.; Chaung, C.K.; Li, W.J.; Chen, T.J.; Chen, B.Y. Electrically tunable liquid-crystal-core optical channel waveguide. J. Light. Technol. 2013, 31, 3570–3574. [Google Scholar] [CrossRef]
- Pham, Q.T.; Nguyen, V.T.; Nguyen, H.T.; Nguyen, M.N. Low-melting point eutectic mixture of two bismaleimides containing polymethylene flexible and aramide-arylate mesogen groups. J. Polym. Res. 2022, 29, 45. [Google Scholar] [CrossRef]
- Srivastava, S.L.; Dhar, R.; Mukherjee, A. Thermodynamical Properties of Bicomponent Mixtures of Liquid Crystals Cholesteryl Pelargonate and Nonyloxybenzoic Acid. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Crys. Liq. Cryst. 2006, 287, 139–154. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Ahmed, H.A.; Hagar, M.; Saad, G.R.; Abu Al-Ola, K.A.; Naoum, M.M. Thermal and Photophysical Studies of Binary Mixtures of Liquid Crystal with Different Geometrical Mesogens. Crystals 2020, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Khalaji, A.D.; Rad, S.M.; Grivani, G.; Das, D. Nickel(II) and copper(II) complexes with an asymmetric bidentate Schiff-base ligand derived from furfurylamine. J. Therm. Anal. Calorim. 2010, 103, 747–751. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Salehifard, M.; Abnosi, M.H. Synthesis, characterization, biological and thermal studies of Cu(II) complexes of salen and tetrahedrosalen ligands. J. Iran. Chem. Soc. 2009, 6, 300–309. [Google Scholar] [CrossRef]
- Caruso, U.; Roviello, A.; Sirigu, A. The liquid-crystalline properties of bis[N-[[4-[4-(alkoxy)benzoyloxy]2-hydroxyphenyl]methylene]alkanamino] complexes of Cu(II), Pd(II) and Ni(II). A general view. Liq. Cryst. 1991, 10, 85–93. [Google Scholar] [CrossRef]
- Marcos, M.; Romero, P.; Serrano, J.-L. Nematic liquid crystal materials containing nickel(II) or copper(II) atoms. Two different kinds of magnetic behaviour. J. Chem. Soc. Chem. Commun. 1989, 1641–1643. [Google Scholar] [CrossRef]
- Van der Veen, J. Influence of terminal substituents upon the nematic-isotropic transition temperature. J. Phys. (Paris) Colloq. 1975, 36, 375–377. [Google Scholar] [CrossRef]
- Gray, G.W.; Winsor, P.A. (Eds.) Ellis Horwood Series in Physical Chemistry: Liquid Crystals and Plastic Crystals, Volume 2: Physico-Chemical Properties and Methods of Investigation; Halsted: Ultimo, Australia, 1974; p. 314. [Google Scholar]
- Naoum, M.M.; Saad, G.R.; Nessim, R.I.; Abdel-Aziz, T.A.; Seliger, H. Effect of molecular structure on the phase behaviour of some liquid crystalline compounds and their binary mixtures II. 4-Hexadecyloxyphenyl arylates and aryl 4-hexadecyloxy benzoates. Liq. Cryst. 1997, 23, 789–795. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Chaturvedi, S.; Tiwari, S.N.; Singh, N.B. Phase diagram of bicomponent mixtures of liquid crystals: Cholesteryl myristate and decyloxybenzoic acid. Emerg. Mater. Res. 2012, 1, 164–167. [Google Scholar] [CrossRef]
- Osman, M.A. Molecular Structure and Mesomorphic Properties of Thermotropic Liquid Crystals. II. Terminal Substituents. Z. Nat. A 1983, 38, 779–787. [Google Scholar] [CrossRef]
- Caruso, U.; Roviello, A.; Sirigu, A. Nematic and smectic mesophases in a new series of Cu(II) metallorganic complexes. II. Liq. Cryst. 1990, 7, 431–438. [Google Scholar] [CrossRef]
- Berthe, M.-C.; Fort, Y.; Caubère, P. A New Synthesis of Thiocyanatoalkyl (Meth)Acrylic Esters. Synth. Commun. 1992, 22, 617–628. [Google Scholar] [CrossRef]
- Singh, S. Impact of Dispersion of Nanoscale Particles on the Properties of Nematic Liquid Crystals. Crystals 2019, 9, 475. [Google Scholar] [CrossRef]









| Compound | Tm 1 | Ti 2 |
|---|---|---|
| 12-8NCu | 103.5 | 129.0 |
| A6O-8NCu | 81.6 | 116.0 |
| A11O-6ONNi | 106.7 | 124.2 |
| A11O-6ONPd | 111.9 c | 143.6 |
| 12-8NCu (%wt.) | Tm 1 | Ti 2 |
|---|---|---|
| 0 | 81.6 | 116.0 |
| 25 | 71.2 | 117.7 |
| 50 | 80.8 | 122.0 |
| 75 | 90.8 | 124.4 |
| 100 | 103.5 | 129.0 |
| A11O-6ONNi (%wt.) | Tm 1 | Ti 2 |
|---|---|---|
| 0 | 111.9 | 143.6 |
| 20.7 | 97.8 | 138.9 |
| 40.1 | 88.7 | 135.2 |
| 60.6 | 94.0 | 131.5 |
| 81.2 | 101.7 | 127.1 |
| 100 | 106.7 | 124.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakemi, H.-A.; Roviello, V.; Caruso, U. Optical and Thermal Investigations of Eutectic Metallomesogen Mixtures Based on Salicylaldiaminates Metal Complexes with a Large Nematic Stability Range. Inorganics 2023, 11, 32. https://doi.org/10.3390/inorganics11010032
Hakemi H-A, Roviello V, Caruso U. Optical and Thermal Investigations of Eutectic Metallomesogen Mixtures Based on Salicylaldiaminates Metal Complexes with a Large Nematic Stability Range. Inorganics. 2023; 11(1):32. https://doi.org/10.3390/inorganics11010032
Chicago/Turabian StyleHakemi, Hassan-Ali, Valentina Roviello, and Ugo Caruso. 2023. "Optical and Thermal Investigations of Eutectic Metallomesogen Mixtures Based on Salicylaldiaminates Metal Complexes with a Large Nematic Stability Range" Inorganics 11, no. 1: 32. https://doi.org/10.3390/inorganics11010032