Experimental Tumor Induction and Evaluation of Its Treatment in the Chicken Embryo Chorioallantoic Membrane Model: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Eligibility and Data Sources
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
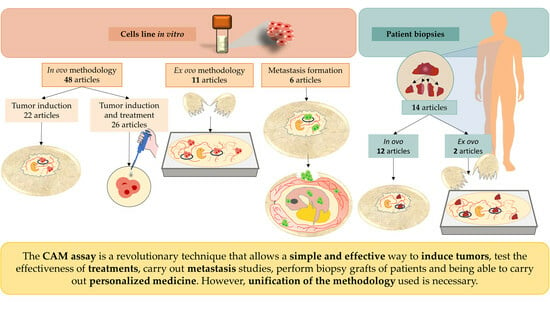
3. Results
3.1. Establishment and Tumor Formation in the CAM from Cell Lines following the In Ovo Methodology
3.2. Tumor Induction Model in the CAM Using the In Ovo Technique to Determine the Effectiveness of Treatments
3.3. Effectiveness of Tumor Induction following the Ex Ovo Methodology
3.4. Use of the CAM Assay as a Model for Metastatic Induction and to Study the Effectiveness of Treatments
3.5. Efficacy of the Patient-Derived Xenograft Model and Its Use to Predict Chemotherapeutic Drug Sensitivity/Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, K.; Yatsyna, A.; Charbonneau, M.; Brochu-Gaudreau, K.; Perreault, A.; Jeldres, C.; McDonald, P.P.; Dubois, C.M. The Chicken Chorioallantoic Membrane Tumor Assay as a Relevant In Vivo Model to Study the Impact of Hypoxia on Tumor Progression and Metastasis. Cancers 2021, 13, 1093. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.a.H.; Glennie, M.J.; et al. Guidelines for the Welfare and Use of Animals in Cancer Research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Chick Embryo Chorioallantoic Membrane Model Systems to Study and Visualize Human Tumor Cell Metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM) Assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Kue, C.S.; Tan, K.Y.; Lam, M.L.; Lee, H.B. Chick Embryo Chorioallantoic Membrane (CAM): An Alternative Predictive Model in Acute Toxicological Studies for Anti-Cancer Drugs. Exp. Anim. 2015, 64, 129–138. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM). A Multifaceted Experimental Model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. In Vivo Models of Angiogenesis. J. Cell Mol. Med. 2006, 10, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Durupt, F.; Koppers-Lalic, D.; Balme, B.; Budel, L.; Terrier, O.; Lina, B.; Thomas, L.; Hoeben, R.C.; Rosa-Calatrava, M. The Chicken Chorioallantoic Membrane Tumor Assay as Model for Qualitative Testing of Oncolytic Adenoviruses. Cancer Gene Ther. 2012, 19, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Taizi, M.; Deutsch, V.R.; Leitner, A.; Ohana, A.; Goldstein, R.S. A Novel and Rapid in Vivo System for Testing Therapeutics on Human Leukemias. Exp. Hematol. 2006, 34, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Koshida, K.; Endo, Y.; Imao, T.; Uchibayashi, T.; Sasaki, T.; Namiki, M. A Chick Embryo Model for Metastatic Human Prostate Cancer. Eur. Urol. 1998, 34, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sys, G.M.L.; Lapeire, L.; Stevens, N.; Favoreel, H.; Forsyth, R.; Bracke, M.; De Wever, O. The In Ovo CAM-Assay as a Xenograft Model for Sarcoma. J. Vis. Exp. 2013, 77, 50522. [Google Scholar] [CrossRef]
- Mangir, N.; Dikici, S.; Claeyssens, F.; MacNeil, S. Using Ex Ovo Chick Chorioallantoic Membrane (CAM) Assay To Evaluate the Biocompatibility and Angiogenic Response to Biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-Step Guide on How to Design, Conduct, and Successfully Publish a Systematic Review and Meta-Analysis in Medical Research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Weighted Kappa: Nominal Scale Agreement Provision for Scaled Disagreement or Partial Credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Pomraenke, M.; Bolney, R.; Winkens, T.; Perkas, O.; Pretzel, D.; Theis, B.; Greiser, J.; Freesmeyer, M. A Novel Breast Cancer Xenograft Model Using the Ostrich Chorioallantoic Membrane-A Proof of Concept. Vet. Sci. 2023, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Gečys, D.; Skredėnienė, R.; Gečytė, E.; Kazlauskas, A.; Balnytė, I.; Jekabsone, A. Adipose Tissue-Derived Stem Cell Extracellular Vesicles Suppress Glioblastoma Proliferation, Invasiveness and Angiogenesis. Cells 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Waltera, A.; Schulz, D.; Schaefer, N.; Stoeckl, S.; Pion, E.; Haerteis, S.; Reichert, T.E.; Ettl, T.; Bauer, R.J. Opposing MMP-9 Expression in Mesenchymal Stromal Cells and Head and Neck Tumor Cells after Direct 2D and 3D Co-Culture. Int. J. Mol. Sci. 2023, 24, 1293. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Heuberger, D.M.; Kivrak Pfiffner, F.; Wolint, P.; Jang, J.-H.; Jungraithmayr, W.; Giovanoli, P.; Calcagni, M.; Waschkies, C.F. Probing Vasoreactivity and Hypoxic Phenotype in Different Tumor Grafts Grown on the Chorioallantoic Membrane of the Chicken Embryo In Ovo Using MRI. Cancers 2022, 14, 3114. [Google Scholar] [CrossRef] [PubMed]
- Doege, A.; Steens, R.; Dünker, N.; Busch, M.A. Retinoblastoma Cell Growth In Vitro and Tumor Formation In Ovo-Influence of Different Culture Conditions. Methods Protoc. 2022, 5, 21. [Google Scholar] [CrossRef]
- Al-Akhrass, H.; Pietilä, M.; Lilja, J.; Vesilahti, E.-M.; Anttila, J.M.; Haikala, H.M.; Munne, P.M.; Klefström, J.; Peuhu, E.; Ivaska, J. Sortilin-Related Receptor Is a Druggable Therapeutic Target in Breast Cancer. Mol. Oncol. 2022, 16, 116–129. [Google Scholar] [CrossRef]
- Aaltonen, N.; Kyykallio, H.; Tollis, S.; Capra, J.; Hartikainen, J.M.; Matilainen, J.; Oikari, S.; Rilla, K. MCF10CA Breast Cancer Cells Utilize Hyaluronan-Coated EV-Rich Trails for Coordinated Migration. Front. Oncol. 2022, 12, 869417. [Google Scholar] [CrossRef] [PubMed]
- Yart, L.; Bastida-Ruiz, D.; Allard, M.; Dietrich, P.-Y.; Petignat, P.; Cohen, M. Linking Unfolded Protein Response to Ovarian Cancer Cell Fusion. BMC Cancer 2022, 22, 622. [Google Scholar] [CrossRef] [PubMed]
- Löffler, J.; Hamp, C.; Scheidhauer, E.; Di Carlo, D.; Solbach, C.; Abaei, A.; Hao, L.; Glatting, G.; Beer, A.J.; Rasche, V.; et al. Comparison of Quantification of Target-Specific Accumulation of [18F]F-siPSMA-14 in the HET-CAM Model and in Mice Using PET/MRI. Cancers 2021, 13, 4007. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, M.; Chua, P.J.; Muniasamy, U.; Huang, R.Y.J.; Thike, A.A.; Ng, C.T.; Tan, P.H.; Yip, G.W.; Bay, B.H. Prognostic Significance of Phosphoglycerate Dehydrogenase in Breast Cancer. Breast Cancer Res. Treat. 2021, 186, 655–665. [Google Scholar] [CrossRef]
- Arlt, A.; von Bonin, F.; Rehberg, T.; Perez-Rubio, P.; Engelmann, J.C.; Limm, K.; Reinke, S.; Dullin, C.; Sun, X.; Specht, R.; et al. High CD206 Levels in Hodgkin Lymphoma-Educated Macrophages Are Linked to Matrix-Remodeling and Lymphoma Dissemination. Mol. Oncol. 2020, 14, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Eckrich, J.; Kugler, P.; Buhr, C.R.; Ernst, B.P.; Mendler, S.; Baumgart, J.; Brieger, J.; Wiesmann, N. Monitoring of Tumor Growth and Vascularization with Repetitive Ultrasonography in the Chicken Chorioallantoic-Membrane-Assay. Sci. Rep. 2020, 10, 18585. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Klein, S.; Große-Kreul, J.; Scheiner, O.; Metz, K.; Stephan, H.; Dünker, N. P53, miR-34a and EMP1-Newly Identified Targets of TFF3 Signaling in Y79 Retinoblastoma Cells. Int. J. Mol. Sci. 2019, 20, 4129. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Große-Kreul, J.; Wirtz, J.J.; Beier, M.; Stephan, H.; Royer-Pokora, B.; Metz, K.; Dünker, N. Reduction of the Tumorigenic Potential of Human Retinoblastoma Cell Lines by TFF1 Overexpression Involves P53/Caspase Signaling and miR-18a Regulation. Int. J. Cancer 2017, 141, 549–560. [Google Scholar] [CrossRef]
- Avram, S.; Coricovac, D.-E.; Pavel, I.Z.; Pinzaru, I.; Ghiulai, R.; Baderca, F.; Soica, C.; Muntean, D.; Branisteanu, D.E.; Spandidos, D.A.; et al. Standardization of A375 Human Melanoma Models on Chicken Embryo Chorioallantoic Membrane and Balb/c Nude Mice. Oncol. Rep. 2017, 38, 89–99. [Google Scholar] [CrossRef]
- Zuo, Z.; Syrovets, T.; Wu, Y.; Hafner, S.; Vernikouskaya, I.; Liu, W.; Ma, G.; Weil, T.; Simmet, T.; Rasche, V. The CAM Cancer Xenograft as a Model for Initial Evaluation of MR Labelled Compounds. Sci. Rep. 2017, 7, 46690. [Google Scholar] [CrossRef]
- Große-Kreul, J.; Busch, M.; Winter, C.; Pikos, S.; Stephan, H.; Dünker, N. Forced Trefoil Factor Family Peptide 3 (TFF3) Expression Reduces Growth, Viability, and Tumorigenicity of Human Retinoblastoma Cell Lines. PLoS ONE 2016, 11, e0163025. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Syrovets, T.; Genze, F.; Abaei, A.; Ma, G.; Simmet, T.; Rasche, V. High-Resolution MRI Analysis of Breast Cancer Xenograft on the Chick Chorioallantoic Membrane. NMR Biomed. 2015, 28, 440–447. [Google Scholar] [CrossRef]
- Urbańska, K.; Sokołowska, J.; Szmidt, M.; Sysa, P. Proliferative and Apoptotic Activity of Glioblastoma Multiforme Cells Cultured on in Ovo Model. In Vivo 2014, 28, 541–548. [Google Scholar] [PubMed]
- Jaworski, S.; Sawosz, E.; Grodzik, M.; Kutwin, M.; Wierzbicki, M.; Włodyga, K.; Jasik, A.; Reichert, M.; Chwalibog, A. Comparison of Tumour Morphology and Structure from U87 and U118 Glioma Cells Cultured on Chicken Embryo Chorioallantoic Membrane. J. Vet. Res. 2013, 57, 593–598. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick Chorioallantoic Membrane (CAM) Assay as an in Vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Balke, M.; Neumann, A.; Kersting, C.; Agelopoulos, K.; Gebert, C.; Gosheger, G.; Buerger, H.; Hagedorn, M. Morphologic Characterization of Osteosarcoma Growth on the Chick Chorioallantoic Membrane. BMC Res. Notes 2010, 3, 58. [Google Scholar] [CrossRef] [PubMed]
- Shoji, C.; Kikuchi, K.; Yoshida, H.; Miyachi, M.; Yagyu, S.; Tsuchiya, K.; Nakaya, T.; Hosoi, H.; Iehara, T. In Ovo Chorioallantoic Membrane Assay as a Xenograft Model for Pediatric Rhabdomyosarcoma. Oncol. Rep. 2023, 49, 76. [Google Scholar] [CrossRef] [PubMed]
- Mitrevska, K.; Merlos Rodrigo, M.A.; Cernei, N.; Michalkova, H.; Splichal, Z.; Hynek, D.; Zitka, O.; Heger, Z.; Kopel, P.; Adam, V.; et al. Chick Chorioallantoic Membrane (CAM) Assay for the Evaluation of the Antitumor and Antimetastatic Activity of Platinum-Based Drugs in Association with the Impact on the Amino Acid Metabolism. Mater. Today Bio 2023, 19, 100570. [Google Scholar] [CrossRef]
- Li, J.; Brachtlova, T.; van der Meulen-Muileman, I.H.; Kleerebezem, S.; Liu, C.; Li, P.; van Beusechem, V.W. Human Non-Small Cell Lung Cancer-Chicken Embryo Chorioallantoic Membrane Tumor Models for Experimental Cancer Treatments. Int. J. Mol. Sci. 2023, 24, 15425. [Google Scholar] [CrossRef]
- Kiening, M.; Lange, N. A Step Ahead to Enhancing Routine Breast Cancer Resection: Spheroid and Hen’s Egg Chorioallantoic Membrane Models to Assess the Photodynamic Diagnosis Efficiency of ALA and PSI-ALA-Hex. J. Photochem. Photobiol. B 2023, 244, 112717. [Google Scholar] [CrossRef]
- Daniluk, K.; Lange, A.; Wójcik, B.; Zawadzka, K.; Bałaban, J.; Kutwin, M.; Jaworski, S. Effect of Melittin Complexes with Graphene and Graphene Oxide on Triple-Negative Breast Cancer Tumors Grown on Chicken Embryo Chorioallantoic Membrane. Int. J. Mol. Sci. 2023, 24, 8388. [Google Scholar] [CrossRef]
- Berner, J.; Miebach, L.; Herold, L.; Höft, H.; Gerling, T.; Mattern, P.; Bekeschus, S. Gas Flow Shaping via Novel Modular Nozzle System (MoNoS) Augments kINPen-Mediated Toxicity and Immunogenicity in Tumor Organoids. Cancers 2023, 15, 1254. [Google Scholar] [CrossRef] [PubMed]
- Sarogni, P.; Mapanao, A.K.; Gonnelli, A.; Ermini, M.L.; Marchetti, S.; Kusmic, C.; Paiar, F.; Voliani, V. Chorioallantoic Membrane Tumor Models Highlight the Effects of Cisplatin Compounds in Oral Carcinoma Treatment. iScience 2022, 25, 103980. [Google Scholar] [CrossRef] [PubMed]
- Swadi, R.; Mather, G.; Pizer, B.L.; Losty, P.D.; See, V.; Moss, D. Optimising the Chick Chorioallantoic Membrane Xenograft Model of Neuroblastoma for Drug Delivery. BMC Cancer 2018, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Lai, D.V.; Wu, W.; Webb, Z.D.; Yang, Q.; Zhao, L.; Yu, Z.; Thorpe, J.E.; Disch, B.C.; Ihnat, M.A.; et al. Transition from Androgenic to Neurosteroidal Action of 5α-Androstane-3α, 17β-Diol through the Type A γ-Aminobutyric Acid Receptor in Prostate Cancer Progression. J. Steroid Biochem. Mol. Biol. 2018, 178, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Rajendran, S.K.; Peuhu, E.; Alshatwi, A.A.; Akbarsha, M.A.; Hietanen, S.; Eriksson, J.E. Novel Action Modality of the Diterpenoid Anisomelic Acid Causes Depletion of E6 and E7 Viral Oncoproteins in HPV-Transformed Cervical Carcinoma Cells. Biochem. Pharmacol. 2014, 89, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Grodzik, M.; Sawosz, E.; Wierzbicki, M.; Orlowski, P.; Hotowy, A.; Niemiec, T.; Szmidt, M.; Mitura, K.; Chwalibog, A. Nanoparticles of Carbon Allotropes Inhibit Glioblastoma Multiforme Angiogenesis in Ovo. Int. J. Nanomed. 2011, 6, 3041–3048. [Google Scholar] [CrossRef]
- Rupp, T.; Legrand, C.; Hunault, M.; Genest, L.; Babin, D.; Froget, G.; Castagné, V. A Face-To-Face Comparison of Tumor Chicken Chorioallantoic Membrane (TCAM) In Ovo with Murine Models for Early Evaluation of Cancer Therapy and Early Drug Toxicity. Cancers 2022, 14, 3548. [Google Scholar] [CrossRef] [PubMed]
- Che, P.P.; Mapanao, A.K.; Gregori, A.; Ermini, M.L.; Zamborlin, A.; Capula, M.; Ngadimin, D.; Slotman, B.J.; Voliani, V.; Sminia, P.; et al. Biodegradable Ultrasmall-in-Nano Architectures Loaded with Cisplatin Prodrug in Combination with Ionizing Radiation Induces DNA Damage and Apoptosis in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 3034. [Google Scholar] [CrossRef]
- Hermann, C.; Lang, S.; Popp, T.; Hafner, S.; Steinritz, D.; Rump, A.; Port, M.; Eder, S. Bardoxolone-Methyl (CDDO-Me) Impairs Tumor Growth and Induces Radiosensitization of Oral Squamous Cell Carcinoma Cells. Front. Pharmacol. 2020, 11, 607580. [Google Scholar] [CrossRef]
- Borugă, M. Assessment of olive leaves extract—Cytotoxicity in vitro and angiogenesis in ovo. Farmacia 2021, 69, 38–43. [Google Scholar] [CrossRef]
- Marcion, G.; Hermetet, F.; Neiers, F.; Uyanik, B.; Dondaine, L.; Dias, A.M.M.; Da Costa, L.; Moreau, M.; Bellaye, P.-S.; Collin, B.; et al. Nanofitins Targeting Heat Shock Protein 110: An Innovative Immunotherapeutic Modality in Cancer. Int. J. Cancer 2021, 148, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Merlos Rodrigo, M.A.; Casar, B.; Michalkova, H.; Jimenez Jimenez, A.M.; Heger, Z.; Adam, V. Extending the Applicability of In Ovo and Ex Ovo Chicken Chorioallantoic Membrane Assays to Study Cytostatic Activity in Neuroblastoma Cells. Front. Oncol. 2021, 11, 707366. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.R.; Rebelo, R.; Rodrigues, J.M.; Xavier, C.P.R.; Vasconcelos, M.H.; Queiroz, M.-J.R.P. Synthesis of Novel Methyl 3-(Hetero)Arylthieno[3,2-b]Pyridine-2-Carboxylates and Antitumor Activity Evaluation: Studies In Vitro and In Ovo Grafts of Chick Chorioallantoic Membrane (CAM) with a Triple Negative Breast Cancer Cell Line. Molecules 2021, 26, 1594. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon Balm Extracts Prevent Breast Cancer Progression In Vitro and In Ovo on Chorioallantoic Membrane Assay. Evid. Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef] [PubMed]
- Achkar, I.W.; Kader, S.; Dib, S.S.; Junejo, K.; Al-Bader, S.B.; Hayat, S.; Bhagwat, A.M.; Rousset, X.; Wang, Y.; Viallet, J.; et al. Metabolic Signatures of Tumor Responses to Doxorubicin Elucidated by Metabolic Profiling in Ovo. Metabolites 2020, 10, 268. [Google Scholar] [CrossRef]
- Waschkies, C.F.; Pfiffner, F.K.; Heuberger, D.M.; Schneider, M.A.; Tian, Y.; Wolint, P.; Calcagni, M.; Giovanoli, P.; Buschmann, J. Tumor Grafts Grown on the Chicken Chorioallantoic Membrane Are Distinctively Characterized by MRI under Functional Gas Challenge. Sci. Rep. 2020, 10, 7505. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and In Ovo Assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef]
- Skarbek, C.; Serra, S.; Maslah, H.; Rascol, E.; Labruère, R. Arylboronate Prodrugs of Doxorubicin as Promising Chemotherapy for Pancreatic Cancer. Bioorg Chem. 2019, 91, 103158. [Google Scholar] [CrossRef]
- Vellanki, S.H.; Cruz, R.G.B.; Richards, C.E.; Smith, Y.E.; Hudson, L.; Jahns, H.; Hopkins, A.M. Antibiotic Tetrocarcin-A Down-Regulates JAM-A, IAPs and Induces Apoptosis in Triple-Negative Breast Cancer Models. Anticancer. Res. 2019, 39, 1197–1204. [Google Scholar] [CrossRef]
- Marinho, A.T.; Lu, H.; Pereira, S.A.; Monteiro, E.; Gabra, H.; Recchi, C. Anti-Tumorigenic and Platinum-Sensitizing Effects of Apolipoprotein A1 and Apolipoprotein A1 Mimetic Peptides in Ovarian Cancer. Front. Pharmacol. 2018, 9, 1524. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Muenzner, J.K.; Caliskan, A.; Ndreshkjana, B.; Erlenbach-Wünsch, K.; Merkel, S.; Croner, R.; Rau, T.T.; Geppert, C.I.; Hartmann, A.; et al. Loss of Enhancer of Zeste Homologue 2 (EZH2) at Tumor Invasion Front Is Correlated with Higher Aggressiveness in Colorectal Cancer Cells. J. Cancer Res. Clin. Oncol. 2019, 145, 2227–2240. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.V.; Berlow, N.E.; Price, L.H.; Mansoor, A.; Cairo, S.; Rugonyi, S.; Keller, C. Preclinical Therapeutics Ex Ovo Quail Eggs as a Biomimetic Automation-Ready Xenograft Platform. Sci. Rep. 2021, 11, 23302. [Google Scholar] [CrossRef] [PubMed]
- Mikulčić, M.; Tabrizi-Wizsy, N.G.; Bernhart, E.M.; Asslaber, M.; Trummer, C.; Windischhofer, W.; Sattler, W.; Malle, E.; Hrzenjak, A. 15d-PGJ2 Promotes ROS-Dependent Activation of MAPK-Induced Early Apoptosis in Osteosarcoma Cell In Vitro and in an Ex Ovo CAM Assay. Int. J. Mol. Sci. 2021, 22, 11760. [Google Scholar] [CrossRef] [PubMed]
- Mélin, C.; Perraud, A.; Christou, N.; Bibes, R.; Cardot, P.; Jauberteau, M.-O.; Battu, S.; Mathonnet, M. New Ex-Ovo Colorectal-Cancer Models from Different SdFFF-Sorted Tumor-Initiating Cells. Anal. Bioanal. Chem. 2015, 407, 8433–8443. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Kats, D.; Rasmussen, S.; Martin, L.R.; Karki, A.; Keller, C.; Berlow, N.E. Design Considerations of an IL13Rα2 Antibody-Drug Conjugate for Diffuse Intrinsic Pontine Glioma. Acta Neuropathol. Commun. 2021, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Buríková, M.; Bilčík, B.; Máčajová, M.; Výboh, P.; Bizik, J.; Mateašík, A.; Miškovský, P.; Čavarga, I. Hypericin Fluorescence Kinetics in the Presence of Low Density Lipoproteins: Study on Quail CAM Assay for Topical Delivery. Gen. Physiol. Biophys. 2016, 35, 459–468. [Google Scholar] [CrossRef]
- Willenbacher, E.; Jöhrer, K.; Willenbacher, W.; Flögel, B.; Greil, R.; Kircher, B. Pixantrone Demonstrates Significant in Vitro Activity against Multiple Myeloma and Plasma Cell Leukemia. Ann. Hematol. 2019, 98, 2569–2578. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ghosh, S.; Banerjee, B.; Santra, A.; Adhikary, A.; Misra, A.K.; Sen, P.C. Phemindole, a Synthetic Di-Indole Derivative Maneuvers the Store Operated Calcium Entry (SOCE) to Induce Potent Anti-Carcinogenic Activity in Human Triple Negative Breast Cancer Cells. Front. Pharmacol. 2016, 7, 114. [Google Scholar] [CrossRef]
- Ademi, H.; Shinde, D.A.; Gassmann, M.; Gerst, D.; Chaachouay, H.; Vogel, J.; Gorr, T.A. Targeting Neovascularization and Respiration of Tumor Grafts Grown on Chick Embryo Chorioallantoic Membranes. PLoS ONE 2021, 16, e0251765. [Google Scholar] [CrossRef]
- Charbonneau, M.; Harper, K.; Brochu-Gaudreau, K.; Perreault, A.; Roy, L.-O.; Lucien, F.; Tian, S.; Fortin, D.; Dubois, C.M. The Development of a Rapid Patient-Derived Xenograft Model to Predict Chemotherapeutic Drug Sensitivity/Resistance in Malignant Glial Tumors. Neuro Oncol. 2023, 25, 1605–1616. [Google Scholar] [CrossRef]
- Golan, T.; Atias, D.; Barshack, I.; Avivi, C.; Goldstein, R.S.; Berger, R. Ascites-Derived Pancreatic Ductal Adenocarcinoma Primary Cell Cultures as a Platform for Personalised Medicine. Br. J. Cancer 2014, 110, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; Taylor, A.; Murray, P.; Poptani, H.; Sée, V. Magnetic Resonance Imaging for Characterization of a Chick Embryo Model of Cancer Cell Metastases. Mol. Imaging 2018, 17, 1536012118809585. [Google Scholar] [CrossRef] [PubMed]
- Nedaeinia, R.; Sharifi, M.; Avan, A.; Kazemi, M.; Nabinejad, A.; Ferns, G.A.; Ghayour-Mobarhan, M.; Salehi, R. Inhibition of microRNA-21 via Locked Nucleic Acid-Anti-miR Suppressed Metastatic Features of Colorectal Cancer Cells through Modulation of Programmed Cell Death 4. Tumour Biol. 2017, 39, 1010428317692261. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, P.; Tayoun, T.; Oulhen, M.; Faugeroux, V.; Rouffiac, V.; Aberlenc, A.; Pommier, A.L.; Honore, A.; Marty, V.; Bawa, O.; et al. Exploitation of the Chick Embryo Chorioallantoic Membrane (CAM) as a Platform for Anti-Metastatic Drug Testing. Sci. Rep. 2020, 10, 16876. [Google Scholar] [CrossRef] [PubMed]
- Desette, A.; Guichet, P.-O.; Emambux, S.; Masliantsev, K.; Cortes, U.; Ndiaye, B.; Milin, S.; George, S.; Faigner, M.; Tisserand, J.; et al. Deciphering Brain Metastasis Stem Cell Properties From Colorectal Cancer Highlights Specific Stemness Signature and Shared Molecular Features. Cell Mol. Gastroenterol. Hepatol. 2023, 16, 757–782. [Google Scholar] [CrossRef] [PubMed]
- Kant, N.; Jayaraj, P.; Sen, S.; Rupani, H.; Kumar, P.; Dahiya, S.; Chugh, P.; Gupta, M.; Sengar, M. Establishment of Patient-Derived Xenografts of Retinoblastoma and Choroidal Melanoma on the Avian Chorioallantoic Membrane. Indian. J. Ophthalmol. 2023, 71, 977–982. [Google Scholar] [CrossRef]
- Tsimpaki, T.; Bechrakis, N.E.; Seitz, B.; Kraemer, M.M.; Liu, H.; Dalbah, S.; Sokolenko, E.; Berchner-Pfannschmidt, U.; Fiorentzis, M. Chick Chorioallantoic Membrane as a Patient-Derived Xenograft Model for Uveal Melanoma: Imaging Modalities for Growth and Vascular Evaluation. Cancers 2023, 15, 1436. [Google Scholar] [CrossRef] [PubMed]
- Rousset, X.; Maillet, D.; Grolleau, E.; Barthelemy, D.; Calattini, S.; Brevet, M.; Balandier, J.; Raffin, M.; Geiguer, F.; Garcia, J.; et al. Embryonated Chicken Tumor Xenografts Derived from Circulating Tumor Cells as a Relevant Model to Study Metastatic Dissemination: A Proof of Concept. Cancers 2022, 14, 4085. [Google Scholar] [CrossRef]
- Pizon, M.; Schott, D.; Pachmann, U.; Schobert, R.; Pizon, M.; Wozniak, M.; Bobinski, R.; Pachmann, K. Chick Chorioallantoic Membrane (CAM) Assays as a Model of Patient-Derived Xenografts from Circulating Cancer Stem Cells (cCSCs) in Breast Cancer Patients. Cancers 2022, 14, 1476. [Google Scholar] [CrossRef]
- Ceaușu, R.A.; Ciolofan, A.; Blidișel, A.; Crețu, O.M.; Cimpean, A.M.; Raica, M. Liver Metastatic Colorectal Tumor Cells Change Their Phenotype During Consecutive Passages on Chick Embryo Chorioallantoic Membrane: Lessons from the Lab to the Clinic. In Vivo 2021, 35, 2711–2718. [Google Scholar] [CrossRef]
- Hu, J.; Ishihara, M.; Chin, A.I.; Wu, L. Establishment of Xenografts of Urological Cancers on Chicken Chorioallantoic Membrane (CAM) to Study Metastasis. Precis. Clin. Med. 2019, 2, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari-Tabrizi-Wizsy, N.; Passegger, C.A.; Nebel, L.; Krismer, F.; Herzer-Schneidhofer, G.; Schwach, G.; Pfragner, R. The Avian Chorioallantoic Membrane as an Alternative Tool to Study Medullary Thyroid Cancer. Endocr. Connect. 2019, 8, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, V.; Mallmann, P.; Petrunkina, A.M.; Rahimi, G.; Nawroth, F.; Hancke, K.; Felberbaum, R.; Genze, F.; Damjanoski, I.; Isachenko, E. Comparison of In Vitro- and Chorioallantoic Membrane (CAM)-Culture Systems for Cryopreserved Medulla-Contained Human Ovarian Tissue. PLoS ONE 2012, 7, e32549. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.; Harper, K.; Brochu-Gaudreau, K.; Perreault, A.; McDonald, P.P.; Ekindi-Ndongo, N.; Jeldres, C.; Dubois, C.M. Establishment of a ccRCC Patient-Derived Chick Chorioallantoic Membrane Model for Drug Testing. Front. Med. 2022, 9, 1003914. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, H.; Wells, G.A.; Miller, M.T.; Villanueva, M.; Pathak, R.; Castro, P.; Ittmann, M.M.; Sikora, A.G.; Lerner, S.P. Characterizing Treatment Resistance in Muscle Invasive Bladder Cancer Using the Chicken Egg Chorioallantoic Membrane Patient-Derived Xenograft Model. Heliyon 2022, 8, e12570. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.T.; Shahin, S.A.; Croissant, J.; Fatieiev, Y.; Matsumoto, K.; Le-Hoang Doan, T.; Yik, T.; Simargi, S.; Conteras, A.; Ratliff, L.; et al. Chick Chorioallantoic Membrane Assay as an in Vivo Model to Study the Effect of Nanoparticle-Based Anticancer Drugs in Ovarian Cancer. Sci. Rep. 2018, 8, 8524. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T. Chick Embryo in Experimental Embryology and More. Pathol. Res. Pract. 2023, 245, 154478. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.; Mesas, C.; Perazzoli, G.; Martínez, R.; Porres, J.M.; Doello, K.; Prados, J.; Melguizo, C.; Cabeza, L. Antiproliferative, Antioxidant, Chemopreventive and Antiangiogenic Potential of Chromatographic Fractions from Anemonia Sulcata with and without Its Symbiont Symbiodinium in Colorectal Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 11249. [Google Scholar] [CrossRef]
- Moeinvaziri, F.; Shojaei, A.; Haghparast, N.; Yakhkeshi, S.; Nemati, S.; Hassani, S.-N.; Baharvand, H. Towards Maturation of Human Otic Hair Cell-like Cells in Pluripotent Stem Cell-Derived Organoid Transplants. Cell Tissue Res. 2021, 386, 321–333. [Google Scholar] [CrossRef]
- Moreno-Jiménez, I.; Hulsart-Billstrom, G.; Lanham, S.A.; Janeczek, A.A.; Kontouli, N.; Kanczler, J.M.; Evans, N.D.; Oreffo, R.O. The Chorioallantoic Membrane (CAM) Assay for the Study of Human Bone Regeneration: A Refinement Animal Model for Tissue Engineering. Sci. Rep. 2016, 6, 32168. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Li, Z.; Lan, Z. Osteogenic effect of collagen/bioglass composites carrying noggin siRNA. Nan Fang. Yi Ke Da Xue Xue Bao 2018, 38, 106–111. [Google Scholar] [PubMed]
- Al-Zghoul, M.B.; Mohammad Saleh, K.M. Effects of Thermal Manipulation of Eggs on the Response of Jejunal Mucosae to Posthatch Chronic Heat Stress in Broiler Chickens. Poult. Sci. 2020, 99, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, E.N.; Enweani, I.B.; Ayanbimpe, G.M. Infection of Chick Chorioallantoic Membrane (CAM) as a Model for the Pathogenesis of Cryptococcus Gattii. Med. Mycol. J. 2018, 59, E25–E30. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Koh, A.P.-F.; Antony, J.; Huang, R.Y.-J. Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies. Cells Tissues Organs 2022, 211, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar] [PubMed]
- Miebach, L.; Berner, J.; Bekeschus, S. In Ovo Model in Cancer Research and Tumor Immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Saller, A.M.; Werner, J.; Süß, S.C.; Reiser, J.; Kollmansperger, S.; Anders, M.; Potschka, H.; Fenzl, T.; Schusser, B.; et al. Nociception in Chicken Embryos, Part I: Analysis of Cardiovascular Responses to a Mechanical Noxious Stimulus. Animals 2023, 13, 2710. [Google Scholar] [CrossRef]
- Bjørnstad, S.; Austdal, L.P.E.; Roald, B.; Glover, J.C.; Paulsen, R.E. Cracking the Egg: Potential of the Developing Chicken as a Model System for Nonclinical Safety Studies of Pharmaceuticals. J. Pharmacol. Exp. Ther. 2015, 355, 386–396. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/Background, Uses, and Future Applications. J. Cell Commun. Signal 2022, 16, 621–626. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Kim, K.; Kang, H. Matrigel Uses in Cell Biology and for the Identification of Thymosin Β4, a Mediator of Tissue Regeneration. Appl. Biol. Chem. 2018, 61, 703–708. [Google Scholar] [CrossRef]








| Ref. | Methodology | Cell Line | Applied Techniques | Results |
|---|---|---|---|---|
| [15] | Day 20: window and tumor induction Day 34: end point by pentobarbital | Breast cancer: MDA-MB-231:
| HE | No tumor growth was observed in the CAM of the ostrich embryo when 1 × 106 cells were inoculated. Fourteen days after inoculation of 2 × 106 cells, tumor growth was observed in two of the ostrich embryos. Inoculation of 6 × 106 tumor cells also did not result in successful tumor formation. Histological analysis showed a diffuse proliferation of epithelial tumor cells within the mesenchymal stroma of the CAM. The tumor cells showed high proliferative activity with numerous mitotic cells. |
| [16] | Day 3: DESH and tumor induction Day 7: sponge implantation Day 12: end point | Glioblastoma:
| HE | Untreated tumors of all cell lines showed diffuse growth. Tumors treated with ASC-EV showed sharper contours and growth on the CAM surface without deep penetration. HE revealed in the control group tumor cell invasion into the mesenchyme of the CAM, ASC-EV treatment reduced tumor invasion of the CAM, and the CAM of treated tumors showed less thickening and fewer blood vessels. |
| [17] | Day 3: DESH Day 7: tumor induction Day 14: end point | BMSCs Squamous cell carcinoma of the head and neck:
Cells were pre-treated with 10 µM of the MMP-9-specific inhibitor JNJ0966 on day 1 and day 3 | - | Eggs whose cells were treated with specific MMP-9 inhibitors showed a significant reduction in tumor size compared to the controls. |
| [18] | Day 3, 5: DESH Day 7: tumor induction Day 14: end point | Lung cancer:
| MRI, IHC | A549 tumors showed a significant T1 response during HCHO. H460 or MC-38 tumors did not respond to HCHO. HIF-1α immunohistochemical staining corroborated the hypoxic phenotype of H460 and MC-38. In addition, ki-67-positive cells were found for H460. |
| [19] | Day 8 and 10: DESH and tumor induction Day 17: end point by decapitation. | Retinoblastoma:
| - | All cell lines showed less tumor formation after inoculation at day 8 compared to inoculation at day 10. Inoculation of different cell concentrations did not change tumor size and weight. Inoculation of 1 × 106 cells is adequate and sufficient for inoculation on day 10, leading to an average size of 6 mm and weight of 34 mg. |
| [20] | Day 3: DESH Day 10: ring and tumor induction Day 16: end point | Breast cancer:
| - | SorLA silencing inhibited tumor growth in both cell lines. |
| [21] | Day 4: DESH Day 8: tumor induction Day 13: end point | Breast cancer:
| IHC, hyaluronan staining | MCF10CA cells showed higher proliferation and formed larger tumors than MCF10A cells. Tumor cell-associated hyaluronan was high in MCF10CA, while MCF10A cells had less intense staining. For ki-67 staining, more positive cells were observed in the MCF10CA line. |
| [22] | Day 8: DESH and tumor induction Day of end point not specific | Ovarian cancer:
| - | No significant differences in tumor size and growth were found in the SKOV3-M line compared to the parents (SKOV—red and SKOV—green). |
| [23] | Day 2: DESH Day 5: place silicone ring Day 6: tumor induction Day 13 and 15: treatment Day of end point not specified | Prostate carcinoma:
| MRI PET HE γ-counter IHC | LNCaP C4-2 reached a volume of 0.025 ± 0.008 mL and PC-3 0.023 ± 0.011 mL for PC-3 after 8 days of growth. HE showed clearly visible tumors in the CAM. PSMA labeling was detected in C4-2 LNCaP tumors, while PC-3 tumors were negative. |
| [24] | Day 3: DESH Day 7: tumor induction Day of end point not specified | Breast cancer:
| Fluorescence | ShPHGDH-C3 and shPHGDH-D4 tumor volumes were smaller than the controls at 24% and 39%, respectively. |
| [25] | Day 3: DESH Day 10: tumor induction Day 14: end point | Hodgkin lymphoma:
| Micro-CT, IHC | The micro-CT assay showed that LM was smaller and without hemorrhages compared to LWM. CD30 revealed that LWM invaded the entire CAM, while LM was compartmentalized in the upper and lower part of the tumor and was absent in the center. Prox1 revealed the absence of lymphatic vessels, while LM presented lymphatic vessels in the area of invasion of the CAM, favoring the diffusion of lymphoma cells. |
| [26] | Day 3: DESH Day 7: tumor induction Day 14: end point by decapitation | Liver cancer:
| Ultrasound, HE | A total of 50.39% of the eggs maintained viability and formed tumors. The tumor volume obtained by ultrasound was 0.69 cm2, and by HE 0.096 cm2, they were significantly correlated. The tumor vascularization obtained by histology and ultrasound was correlated. |
| [27] | Hole opening day not specified Day 10: tumor induction Day 17: end point | Retinoblastoma:
| _ | Tumors from EMP1-overexpressing cells exhibited greater size, weight, and volume than the controls that did not overexpress EMP1. |
| [28] | Hole opening day not specified Day 2: tumor induction Days 10–17: end point | Retinoblastoma:
| FM, RNA extraction | Stable, lentiviral TFF1 overexpression reduces tumor formation capacity of Y79 and RB355 cells in CAM assay results. |
| [29] | Day 4: DESH Day 10: tumor induction Day 18: end point | Melanoma:
| - | Tumor formation was successfully induced from the A375 cell line on the CAM; tumors were compact on day 4 and had sizes of 2.2 ± 0.4 mm2 and 1.5 ± 0.3 mm2, respectively. Furthermore, great angiogenesis was observed around the formed tumors. |
| [30] | Day 4: DESH Day 7: tumor induction Day of end point not specified | Breast cancer:
| MRI | In ovo MRI can be used for assessment of the in vivo biodistribution of labeled compounds, thus enabling efficient non-invasive initial testing. |
| [31] | Day 2: DESH and tumor induction Day 10–17: end point | Retinoblastoma:
| HE | The CAM assays revealed that TFF3 overexpression influences anchorage-independent growth and significantly decreases the size of tumors forming from retinoblastoma cells. |
| [32] | Day 4: DESH Day 7: tumor induction Day 16: end point | Breast cancer:
| MRI | High-resolution magnetic resonance imaging can be used as an effective technique to monitor tumor growth in ovo. |
| [33] | Day 6: DESH and tumor induction Day 18: end point | Glioblastoma:
| HE, IHC, TUNEL | Tumor induction in ovo allows the tumor tissue to maintain the biological characteristics corresponding to primary glioblastoma multiforme. |
| [34] | Hole opening day not specified Day 7: tumor induction Day 17: end point | Glioblastoma:
| HE, TEM | Tumors were successfully induced in the CAM of the egg from glioblastoma cell lines (U87 and U118). |
| [35] | Day 3: DESH Day 11: tumor induction Day 14: end point | Ovarian cancer:
| HE, IHC | The CAM assay is a robust and cost-effective model for the testing of new bioactive antitumor agents, as it is an effective model for the study of ovarian cancer cell metastasis. |
| [36] | Day 3: DESH Day 10: tumor induction Day 17: end point | Different osteosarcoma cell lines at different concentrations. | HE | The CAM assay allows tumor development from osteosarcoma cell lines, making it possible to use it for the preclinical detection of anticancer molecules. |
| Ref. | Methodology | Cell Line | Treatment and Administration | Applied Techniques | Results |
|---|---|---|---|---|---|
| [37] | Day 9: DHES and silicone ring and tumor induction Day 12: treatment Days 14, 16, and 18: end point by overdose of pentobarbital | Rhabdomyosarcoma:
| VCR at 1 nM, 10 nM, and 1 μM Administration: topical | HE IHC | Tumor cells were positive for human-specific vimentin, as confirmed by IHC. Antibodies against human vimentin did not cross-react with chicken tissues. The volume of resected tumors decreased in a concentration-dependent manner. In addition, the necrotic spread was concentration-dependent. |
| [38] | Day 10: DHES and tumor induction Day 16: treatment End point day: not specified | Breast cancer:
| CDDP at 100 μg/mL PtNPs-10 and PtNPs-40 at 250 μg/mL Administration: topical | HE FC | PtNPs-10 achieved greater tumor growth inhibition, while growth inhibition by CDDP was not significant. HE staining showed that cells migrate from the primary tumor, invading the nearby CAM. Tumors treated with CDDP and PtNP showed partial disintegration of the primary tumor. PtNPs-40 induced a visible degradation of the tumor into smaller fragments scattered around the primary tumor. |
| [39] | Day 6: DHES and tumor induction Days 9 and 11: treatment Day 18: end point by freezing at −20 °C overnight | NSCLC:
| Pemetrexed at 1.5 to 20 mg/kg CDDP at 0.1 to 3 mg/kg Pemetrexed at 10 mg/kg with CDDP at 10 mg/kg Administration: not specified | HE IHC bioluminescence | Because of their irregularity in forming tumors, lines SW1573, H1299, and H292 were not chosen for treatment. Line A549 formed solid tumors, while H460 formed less compact tumors. The percentage of Ki-67- and APE1-positive cells in H460 tumors was approximately 100%, while in A549, the majority of cells were Ki-67-negative Combination chemotherapy decreased the tumor in A549, while the size of the treated H460 tumors was not evaluated, possibly due to extensive tumor cell synthesis |
| [40] | Day 8: DHES and tumor induction with spheroid Day 12: treatment Day 14: end point by freezing at −20 °C | Breast cancer:
| ALA and PSI-ALA-Hex 33 at 100 and 300 μmol/kg Administration: intravenous | FC | ALA induced the highest PpIX selectivity at 300 μmol/kg. After day 4 of injection, all spheroids reached maximal selectivity, except MCF-7.ALA, which is able to induce PpIX accumulation in all breast spheroids. PSI-ALA-Hex induced the highest selectivity in all lines at 300 μmol/kg, although it was lower than ALA. |
| [41] | Day 7: DHES, silicone ring, and tumor induction Day 14: treatment Day 17: end point | Breast cancer:
| M at 10 µg/mL GN and GO, MGN and MGO at 20 µg/mL Administration: injection | ELISA lipid peroxidation assay, SOD activity, and GSH-level assay | No significant differences in tumor mass and volume were observed. Significant increase in caspase 3 and 8 in all treatments, with the greatest increase observed with M and MGO treatments. Increase in MDA concentration in the M- and MGN-treated groups and a decrease in the GO-treated group. Increase in SOD activity for all groups compared to the control. Increase in the GSH in the groups treated with M, GN, and GO and a decrease in the groups treated with MGN and MGO. Significant increase in the 8-OHdG marker in the M, GO, MGN, and MGO of the treated groups. |
| [42] | Day 8: DHES and tumor induction Day 12: treatment Day 14: end point | Colorectal carcinoma:
| Gaseous plasma Administration: gas | Luminescence | No difference was found in tumor shrinkage by treatment with gas plasma (kINPen) supplemented with MoNoS adapters at 2 slm relative to argon gas-treated controls. However, at 5 slm, the kINPen treatment caused severe hemorrhage, while the adapters allowed this treatment at 5 slm, and tumor shrinkage was observed. |
| [43] | Day 6: DHES and tumor induction Day 10: treatment Day 14: end point | Squamous cell carcinoma of the head and neck:
| CDDP at 688 μM NAs-cisPT at 688 μM of CDDP and 24 μg Au and NAs 24 μg Administration: not specified | HE | Tumors treated with CDDP and NAs-cisPt showed significant tumor shrinkage, while those treated with NAs showed no significant change in tumor size. NAs-cisPt administration led to a deterioration of the CAM; however, tumors treated with CDDP and NAs had undamaged areas. |
| [44] | Day 3: DHES and tumor induction Days 11–13: treatment Day 14: end point | Neuroblastoma:
| ATRA at 10 μM and 100 μM Administration: injection | PCR IHC | ATRA reduced cell proliferation and promoted a change in differentiation markers. |
| [45] | Day 10: DHES and tumor induction Day 10: treatment Day 12: end point | Prostate cancer:
| 3α-diol at 10−9 M Administration: injection | - | -3α-diol can act as a neurosteroid in PCa cells to activate the GABAAR and may have a role in transforming androgen-dependent to growth factor-dependent pathways for CRPC progression. |
| [46] | Day 8: DHES, polyethylene ring, and tumor induction Days 9–13: treatment Day 14: end point | Cervical cancer:
| AA at 0–20 mg/kg Administration: topical | - | Tumors induced in the CAM from the SiHa cervical cancer cell line treated with anisomelic acid had significantly lower growth than the controls. |
| [47] | Day 6: DHES and tumor induction Day 13: treatment Day 15: end point | Glioblastoma:
| UDD at 500 μg/mL MW-RF at 500 μg/mL Administration: topical | HE PCR | Both types of nanoparticles were effective as they significantly reduced tumor size and angiogenesis. Furthermore, UDD and MW-RF reduced the expression of fibroblast growth factor 2 and vascular endothelial growth factor. |
| [48] | Day 7: DHES, silicon ring, and tumor induction Days 10 and 13: treatment Day 17: end point using 1 mL of 10% formalin for 60 min at room temperature | Colorectal carcinoma:
| CDDP at 0.2, 0.4, 1, 2, 4, and 10 mg/kg Sorafenib at 2 mg/kg Doxorubicin at 0.4 mg/kg Cyclophosphamide at 1, 10, or 100 mg/kg TMZ at 1 mg/kg 5-FU at 1 mg/kg Administration: topical | - | TMZ significantly reduced the weight of glioblastoma (U118MG, GL261) and melanoma tumor (A375). CDDP reduced the weight of PC-3, HCT-116, CT26, A375, and HepG2, but did not affect the weight of PC9/CR tumors. Doxorubicin significantly reduced the weight of mouse 4T1, PC-3, HCT-116, CT26, and HepG2 tumors. 5-FU reduced HCT-116 tumor weight. Sorafenib did not affect the weight of GL261 or A375, and it had a minor effect on HCT-116 tumor weight. |
| [49] | Day 3: DHES Day 6: tumor induction Day 10: treatment Day 17: end point | Ductal adenocarcinoma of the pancreas:
| CDDP at 213 μM USNP at 16.8 μg of Au in NAs-cisPt. Administration: topical The embryos with the combined treatments were subsequently irradiated with 4G and γ | HE | Tumors induced from the PDAC3 tumor line treated with NAs-cisPt significantly reduced their tumor volume. HE staining of SUIT2-028 tumors showed ductal structures typical of PDAC. Tumor weight after NAs-cisPt treatment did not differ from the untreated group; however, the addition of 4G and γ reduced the weight in both the untreated and NAs-cisPt groups. |
| [50] | Day 7: DHES, silicone ring, and tumor induction Day 8: treatment Day 14: end point | Oral Squamous Cell Carcinoma:
| CDDO-Me at 10 nM Administration: topical | HE IHC | CDDO-Me treatment significantly reduced tumor volume. There was no significant difference in Ki-67 expression between the two groups. |
| [51] | Day 4: DHES Day 10: tumor induction Treatment and end point day: not specified | Melanoma:
| Ethanolic extract of olive leaves at 5, 15, and 100 μg/μL Administration: not specified | - | Doses of 15 µg/mL and 100 15 µg/mL of olive leaf extract showed a greater effect on cell growth and the development of narrow vessels in the A376 tumor. |
| [52] | DHES day: not specified Day 9: tumor induction Days 10, 12, 14, 15, and 17: treatment Day 18: end point | Breast cancer:
| A-C2 at 20 mg/kg Anti-PD-L1 nanofitin (B11) at 20 mg/kg A-C2-B11 at 20 mg/kg Pembrolizumab at 2 mg/kg Administration: injection | - | Pembrolizumab, A-C2, and C2-B11 treatments significantly reduced tumor weight. |
| [53] | Day 10: DHES and tumor induction Day 16: treatment Day 17: end point | Neuroblastoma:
| CDDP at 100 µM Elli at 200 µM Administration: topical | qPCR IHC | CDDP and Elli reduced tumor weight compared to the control by 2 and 3.5 times, respectively. CD44 revealed that the primary tumor altered the upper epithelium and invaded the CAM. qPCR analysis of human alu showed that CDDP and Elli reduced the extravasation of tumor cells to the distant CAM and the liver, lungs, and brain. |
| [54] | Day 3: DHES Day 9: nylon ring and tumor induction Day 10: treatment Day 14: end point | Breast cancer:
| 2e at 3 µM Administration: topical | HE | 2e reduced tumor size compared to the control. HE showed the viability of the tumors in both conditions. |
| [55] | Day 4: DHES Day 10: plastic ring, tumor induction, and treatment Day14: end point | Breast cancer:
| Extracts of leaves of Melissa officinalis:
UA at 50 μM Administration: topical | - | The compounds prevented tumor growth outside the ring. MOE96 showed a greater antiproliferative effect and antiangiogenic effects in the MCF7 line after 24 h of treatment, and in the MDA-MB-231 line after 96 h. |
| [56] | Day 9: DHES and tumor induction Days 11, 15, and 17: treatment Day 18: end point | Breast cancer:
| Doxorubicin at 50 μM Administration: topical | Metabolic and lipidomic profiling | Tumors treated with doxorubicin showed smaller sizes and weights. The metabolic and lipid analysis was different in the tumors treated with doxorubicin compared to the controls. Doxorubicin inhibits glycolysis, nucleotide synthesis, choline metabolism, and fatty acid metabolism. On the contrary, antioxidant pathways are activated. |
| [57] | Days 3 to 5: DHES Day 7: plastic ring and tumor induction Day 14: end point | Colorectal cancer:
| Air and carbogen Administration: through plastic tubes to the CAM | MRI (T1 and T2) HE IHC | MC-38 tumors showed volumes 65% larger than A549 tumors. A549 tumors showed significantly higher T2 values and no changes in T1 after exposure to carbogen, while MC-38 tumors showed no changes in T1 and T2. MC-38 tumors showed larger T1 and T2 in the center, while in A549 they were distributed on the surface. MCF-7 tumors showed a greater number of proliferative cells (Ki-67) distributed homogeneously. A549 tumors had fewer proliferative cells distributed irregularly on the surface. Based on HIF-1-α-positive cells, the density of hypoxic cells was higher for MC-38 tumors compared to A549. |
| [58] | Day 4: DHES Day 8: tumor induction Day 11: silicone ring and treatment Days 13 to 15: end point | Ovarian cancer:
| CBD at 100 µM CBD-NP NP Administration: topical | HE | HE revealed that cells invaded the CAM to form the tumor mass. CBD and CBD-NP significantly reduced tumor growth by 1.38 and 1.5 times, respectively. |
| [59] | Day 10: DHES and tumor induction Day 13: treatment Day 15: end point | Pancreatic cancer:
| Doxorubicin at 184 nmol 8a at 184 nmol Administration: Intratumoral injections | HPLC | Doxorubicin and 8a reduced tumor size by 50%, and tumor necrosis was observed. HPLC revealed the same amount of doxorubicin in the tumors (30%), but 8a was not detected. |
| [60] | DHES day: not specified Day 8: tumor induction Day 10: treatment Day 13: end point | Breast cancer:
| Tetrocarcin-A at 2.5 µM Administration: topical | HE IHC | Tetrocarcin A reduced tumor growth compared to the control. Tetrocarcin A-treated tumors showed 60% of cleaved caspase 3 expression compared to the control (10%). |
| [61] | Day 3: DHES Day 9: treatment of cells and after tumor induction Day 16: end point | Ovarian cancer:
| ApoA1 at 100 μg/mL CDDP at 15 μM Treatment with cells | FC | The combination of ApoA1 and CDDP showed a greater reduction in tumor size than both treatments alone. In addition, the combination was synergistic. |
| [62] | Day 8: DHES Day 9: tumor induction with treated cells Day 14: end point | Colorectal cancer:
| DZNep at 5 µM Treated cells prior to tumor induction | HE IHC | DZNep-treated tumors showed smaller sizes and fewer cells in clusters with large areas of Matrigel. However, DZNep-treated tumors also showed a greater capacity for CAM invasion and a higher degree of vascularization, displaying a more aggressive phenotype. DZNep reduced EZH2 expression levels in tumors. High heterogeneity of EZH2 and H3K27me3 staining intensity was detected in DZNep-treated tumors. |
| Ref. | Methodology CAM Assay | Cell Line | Treatment | Applied Techniques | Results |
|---|---|---|---|---|---|
| [38] | The day of inoculation is not indicated. Drug treatment 72 h after inoculation. Euthanasia 24 h after treatment | Breast:
| CDDP: 100 µg/mL, 5 µL (24 h) PtNP-10: 250 µg/mL, 5 µL (24 h) PtNP-40: 250 µg/mL, 5 µL (24 h) | Evaluation of tumor migration and localization in the CAM and embryo by CellTracker Green fluorescence. Tumor area quantification | Reduction in the tumor area in 24 h drug treatments, highlighting the reduction in PtNP-10 treatments. Reduction in CAM metastasis in PtNP treatments. |
| [53] | Day 3: TCE Day 10: tumor induction Day 13: treatment Day 14: euthanasia by incision in the vitelline arteries | Neuroblastoma:
| CDDP: 100 µM, 5 µL (24 h) Elli: 200 µL, 5 µL (24 h) | Cell tracking histology, immunohistochemistry, and qRT-PCR | A tumor was successfully induced from Nbl UFK-NB-4 cells in the CAM. CDDP and Elli treatments eliminated the intravasation and extravasation of UFK-NB-4 cells, as well as dramatically reduced the tumor size. |
| [63] | Day 3: TCE Day 7: tumor induction + treatment Day 11: euthanasia | Osteosarcoma:
| Osteosarcoma: BEZ235: 500 nM (72 h). drug mixed with cellular/hydrogel solution Hepatocarcinoma: Volasertib: 0.3 µM, 3 µM and 30 µM in PBS | Osteosarcoma: Histology and bioluminescence measurement Hepatocarcinoma: luciferase imaging study and cytotoxicity essay | Reduction by 20% of the U48484 fluorescence with BEZ235 treatment. Inhibition of 50% of the tumor growth of HB243 and HB282 treated with voasertib. |
| [64] | Day 3: TCE Day 9: engraftment of pre-treated cells Day 13: euthanasia | Osteosarcoma:
| 15d-PGJ2: 20 µM (24 h). In vitro treatment before engraftment | Tumor area, morphology, and proliferation quantification Immunohistochemistry | Reduction in tumor size and density in U2-OS and Saos-2 lines previously treated. Reduction in the cell proliferation marker Ki-67 in the presence of the drug. |
| [65] | Day 3: TCE Day 10: tumor induction Day 13. euthanasia | Colorectal:
| Previous growth of cancer cells in Matrigel before graft | Histology, immunohistology, Western blot, and stem cell proteome array | Matrigel 3D pre-culture of the tumoral cells facilitates the vascularization of the CAM tumors and promotes differences in proliferation, protein markers, and gene expression among the different treatments. |
| [66] | Day 2 (after 54 h of incubation): TCE Day 7: tumor induction + treatment Day 12: euthanasia | Glioblastoma:
| Anti-IL13Rα2::PBD: 50 ng/mL, 500 ng/mL and 5 µg/mL in 25 µL of PBS; 29 min; in vitro treatment before cell graft | Luminescence measurement with luciferase | Dose–dependent antitumor effect of Anti-IL13Rα2 in the CAM tumor. |
| [67] | Day 3: transfer the contents of the egg into a sterile 6-well plate Day 7: tumor induction Day 8: treatment and euthanasia | Esophageal: TE1: spheroid implantation Incubation of 500 cells in 250 µL per well in 96-well plates. Three to five weeks incubation | Hyp Hyp:LDL 100:1 Hyp:LDL 200:1 79 µM in PBS with 0.17% DMSO (2 µg/ g of embryo) 6 h of incubation | CAM photo analysis using ImageJ software and spectroscopic measurement of fluorescence | Differences in proliferation, protein markers, and gene expression among the different treatments. |
| [68] | Day 3: TCE Day 9: tumor induction + treatment. Day 14: euthanasia | Myeloma: OPM-2: 3 × 105 cells in 30 µL of mouse tail type 1 collagen solution in ×10 of DMEM and neutralized with 0.1 N NaOH | PIX: 1 µM | Fluorescence by the GFP labeling of cells and quantification by ELISA | Blockage of tumor growth in the presence of kinin due to a reduction in tumor area. |
| [69] | The TCE is not indicated. Day 6: tumor induction Day 10: first treatment Day 12: second treatment Day 15: euthanasia | Breast: MDA-MB-231: 2 × 106 cells per CAM | Phemindole: 10 µL/mL | Amplification of the alu sequences of the tumor cells | Suppression of tumor formation with phemindole treatment at a dose of 10 uM. |
| [70] | Day 3: TCE Day 9: tumor induction Day 19: treatment Day 14: euthanasia | Human glioblastoma: U87: 4 × 105 cells per CAM Canine melanoma: 17CM98: 4 × 105 + 1/5 Matrigel per CAM Canine osteosarcoma:
| AVA: 10 mg/kg, intravenous injection; 50 µL CHC: 60 mg/kg, topical application around explant; 50 µL AZD3965: 2.5 µM per egg, intravenous injection; 50 µL AVA + CHC AVA + AZD | Tumor growth, tumor perfusion, and tumor hypoxia | Decrease in tumor size in the presence of the drugs, highlighting the effect of combined treatment in U87. Reduction in tumor perfusion in combined VPA + CHC therapy. Reduction in hypoxia in VPA treatment. |
| [71] | The TCE is not indicated. Day 9 or 10: tumor induction Day 11 or 12 (48 h after induction): treatment Day 16: euthanasia by decapitation | Glioma tissue: 1–2 mm diameter piece, with Matrigel Glioblastoma: U-87: 0.75 × 106 MG: 0.75 × 106 LN-18: 1 × 106 | Carboplatin: 8 mg/kg (48 h) TMZ: 4 mg/kg (48 h) | Histology and immunostaining, genomic analysis, and drug sensibility assays | Tumor-forming capacity in the CAM. Antitumor effect of drugs on tumors formed in the CAM. |
| Ref. | CAM Assay Methodology | Cell Line | Techniques | Results |
|---|---|---|---|---|
| [53] | Day 10: DHES and tumor induction Day 16: treatment with CDDP (100 µM) or Elli (200 µM) topically Day 17: euthanasia | Bone marrow metastases that are high risk:
| Intravasated/extravasated cells; qPCR after DNA extraction for Alu sequences | CDDP and ELLI exhibited significant inhibitory activity against extravasation to the liver, lungs brain, and distal CAM. |
| [62] | Day 8: DHES Day 9: tumor induction Day 14: euthanasia | Colorectal cancer:
| IHC of CAM tumors FFPE; relative vessel density intra- and peri-tumoral | Tumors without EZH2 expression have an increase in the number of vessels and higher tumor aggressiveness. |
| [72] | Day 8: DHES and tumor induction placed in a polypropylene ring Day 16: euthanasia | Pancreatic cancer:
| PCR after DNA extraction from chicken liver | Chicken liver cells have a high expression of CK7. |
| [73] | Day 7: DHES and tumor induction Day 14: euthanasia | Neuroblastoma:
| MRI; frozen tissue slices analyzed with an epi-fluorescent microscope | MRI technique can detect metastasis deposits of up to 12 cells. |
| Day 7: DHES and tumor injection in the chicken brain Day 14: euthanasia | Neuroblastoma:
| |||
| [74] | Day 10: DHES and tumor induction placed in a plastic ring Day 18: anesthesia by ice and euthanasia | Colorectal cancer:
| qPCR after DNA extraction for Alu sequences; transfection | Chicken liver cells have a significantly decreased number of metastases than LNA-anti-miR-21 groups. |
| [75] | Day 3: DHES Day 10: tumor induction Day 11: treatment Day 17: euthanasia | Prostate cancer:
| Fluorescent macroscopy imaging; 3D chick embryo for fluorescence detection; FFPE histochemistry | CDDP and docetaxel treatment decreased the metastatic foci detection. |
| Ref. | Sample | CAM Xenograft Model | Applied Studies | Results |
|---|---|---|---|---|
| [71] | Biopsy from 60 patients with glioma undergoing treatment | Ex ovo Days 8–10: freshly resected tumor implanted (1–2 mm) Two days after implantation: treatment injected into the CAM vasculature CB: 8 mg/Kg and TMZ: 4 mg/Kg Day 16: euthanasia by decapitation | Genomic analysis HE IHC | A total of 98.3% of glioma specimens established xenograft tumors on the CAM. The glioma CAM-PDX model retained the histopathology and molecular characteristics of the original tumor. Higher CAM-PDX tumorigenicity is associated with poorer prognosis in glioma patients. |
| [76] | Isolate CSC from 4 patients with CRC and BM. | In ovo Day 9: DHES and implant 3 × 106 cells Day 18: remove the tumor of the CAM, wash with PBS, and transfer in PFA for 48 h | PCR | Tumor formation was correctly established from the BM-SC-CRC lines. In addition, they acquired invasion and migration capabilities in the CAM. The cell lines from patients 1 and 2 were capable of generating metastasis. |
| [77] | Biopsy of CM (Stage IV) and RB from 2 patients undergoing enucleation | In ovo Day 9: the air sac was punctured and suctioned. DHES and place a sterile silicone o-ring on a visible vascularization area Day 10: three PDX were implanted in the o-ring Day 17: euthanasia by hypothermia | HE IHC | RBs and CM PDXs successfully induced tumors in the CAM. Furthermore, angiogenesis was observed in the tumor and intratumoral periphery. |
| [78] | Biopsy from 6 advanced-stage uveal melanoma patients | In ovo Day 5: remove 4–10 mL albumin Day 6: DHES Day 7: lacerate the surface of the CAM causing bleeding. Three methods of implantation:
Day 18: euthanasia by decapitation | Ultrasound scans Optical tomography Fluorescein angiography HE IHC | Biopsy fragments from patients with uveal melanoma were successfully established in the CAM. Furthermore, the techniques applied in their study allowed the CAM assay to be used as a PDX model in experimental oncology. |
| [79] | CTC of 35 cancer patients (6 prostate, 6 breast, 23 lung) | In ovo Day 9: DHES and place the CTC suspension onto the CAM Day 18: euthanasia | HE IHC PCR NGS analysis | Tumors from biopsies grafted onto the CAM showed genomic concordance with the original patient’s tumor and its liquid biopsy by scanning the DNA sequence using NGS. Furthermore, these results generated a patent: “WO2020/089560A1; 7 May 2020.” |
| [80] | CCSC of 10 women diagnosed with breast cancer in different stages of the disease | In ovo Day 4: DHES Day 8: Engraft tumorospheres of CCSC in Matrigel directly. Day 16: Euthanasia by decapitation. | HE | Histological studies showed that tumors in the CAM maintained the initial structure. Furthermore, biopsies from patients with a high Ki-67 index were the most likely to develop tumors in the CAM membrane. |
| [81] | LM of CR adenocarcinoma biopsy | In ovo Day 3: open DHES Day 8: apply silicone ring and implant the suspension of tumor cells derived from CRLM Day 12: xenograft was transferred to the membrane of another egg Day 16: euthanasia | IHC | Tumor induction in the CAM was successful, generating solid tumors with increased angiogenesis around them from the vascularization of the CAM. Likewise, there was a concordance between the response of the patients and that observed in the tumors in the CAM in terms of aggressiveness and metastasis formation. |
| [82] | Isolate cells from the patient’s ccRCC | Day 7: DHES Day 10: patient tissue-derived primary cancer was implanted cells in Matrigel (2–3 mm) and isolated 2 × 106 cells in Matrigel Day 20: euthanasia by being placed on ice for 20 min | Histology | A high efficiency was obtained in the grafting of PDX in the CAM since it was generated in just 10 days, and it could be a very promising assay to carry out studies on drug detection in the tumor of an individual patient. |
| [83] | MTCs from 2 patients | Ex ovo Day 3: eggshell crack Day 10: silicon ring and implement tumor tissue (2 mm) Day 16: histological analysis | Histology IHC | The samples grafted into the CAM remained alive and also expressed specific neuroendocrine markers synaptophysin and chromogranin A. |
| [84] | Biopsy from 9 patients with ovarian cancer | In ovo Day 4: DHES Day 10: a silicone ring and an ovarian piece were placed into the ring Day 15: survival rate evaluated | Histology IHC | The survival rate of chicken embryos was 97.2%. After tumor induction, there was an increase in angiogenesis around the tumor. |
| [85] | Biopsy from 24 ccRCC patients | In ovo Days 8–10: tumor fragment implanted onto the CAM in Matrigel. 2 days after implantation: treatment with Sunitinib IV. Day 16: euthanasia | HE IHC | It was observed macroscopically that after tumor induction in the CAM, the number of blood vessels around it increased. CAM tumor xenografts from patients with ccRCC retain the histopathological characteristics of the original tumor. |
| [86] | Biopsy of 22 patient with bladder cancer | In ovo Day 7: DHES and place a silicone ring. After 2 h, engraft homogenates from cryopreserved or engraft fresh tumors in Matrigel Day 11: daily treatment with saline and/or DMSO vehicle control; GMZ; cisplatin; afatinib; abemaciclib; AZD4547 Day 18: euthanasia | HE IHC | Primary tumors grafted onto the CAM as ground homogenates adopted a different morphological phenotype compared to the original tumors. Fresh tumors grew well and showed similar histology to the original tumor. Ki-67 expression was better retained in tumors grafted from frozen samples compared to fresh ones. Clinical resistance to cisplatin-based chemotherapy of pre-NAC MIBC tumors was maintained in CAM-PDX. |
| [1] | Three ccRCC patients’ tumor tissue | In ovo Day 9: patients’ tumor fragments with Matrigel Days 10–16: treatment with vehicle or sorafenib Day 16: eggs sacrificed by decapitation | qPCR IHC HE | The treatment of tumors induced in the CAM from patient biopsies showed the same response as patients in the clinic. The xenograft from patient 1 responded, while the xenografts derived from patients 2 and 3 did not respond. |
| [87] | Fresh tumor samples from ovarian cancer patients | In ovo Day 10: DHES and a Teflon ring. Inoculation of tumor Day 13: 0.1 mL of nanoparticle or nanoparticle/doxorubicin solution was injected into the CAM blood vessel Day 19: sacrifice eggs | HE | PMO loaded with doxorubicin decreased tumor volume. All eggs survived the injection of nanoparticles with doses of up to 200 μg of doxorubicin. The internal organs (liver, heart, intestines, kidneys, and spleen) appeared normal. Macroscopically, the tumor was observed four days after transplantation, and the size of the tumor grew exponentially six days later. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesas, C.; Chico, M.A.; Doello, K.; Lara, P.; Moreno, J.; Melguizo, C.; Perazzoli, G.; Prados, J. Experimental Tumor Induction and Evaluation of Its Treatment in the Chicken Embryo Chorioallantoic Membrane Model: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 837. https://doi.org/10.3390/ijms25020837
Mesas C, Chico MA, Doello K, Lara P, Moreno J, Melguizo C, Perazzoli G, Prados J. Experimental Tumor Induction and Evaluation of Its Treatment in the Chicken Embryo Chorioallantoic Membrane Model: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(2):837. https://doi.org/10.3390/ijms25020837
Chicago/Turabian StyleMesas, Cristina, Maria Angeles Chico, Kevin Doello, Patricia Lara, Javier Moreno, Consolación Melguizo, Gloria Perazzoli, and Jose Prados. 2024. "Experimental Tumor Induction and Evaluation of Its Treatment in the Chicken Embryo Chorioallantoic Membrane Model: A Systematic Review" International Journal of Molecular Sciences 25, no. 2: 837. https://doi.org/10.3390/ijms25020837