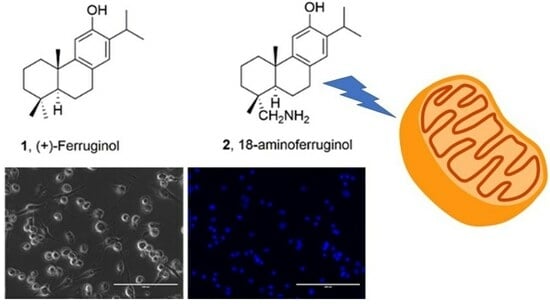
In Vitro Cytotoxic Effects of Ferruginol Analogues in Sk-MEL28 Human Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. In Vitro Antiproliferative Activity of Ferruginol and Analogues
2.2. Changes in Cell Morphology Associated with the Treatment Point Out to the Induction of Apoptosis
2.3. Discerning Early and Late Events of Intrinsic Apoptosis
2.4. Migration Assay and Invasion Assays
3. Discussion
3.1. On the Ferruginol Derivatives Structure-Antiproliferative Activity Relationship
3.2. Introduction of the Amino Group in Position 18 Changes the Dynamics of the Apoptotic Process of the Parent Molecule in SK-MEL-28 Cells
3.3. Effect of Ferruginol Derivatives in Other Cancer Cell Lines: Prospects
3.4. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cell Lines
4.3. Sulforhodamine B (SRB) Assay
4.4. Caspase-3/7 Activity Assay
4.5. Phase-Contrast and Fluorescence Microscopy DAPI Staining
4.6. Determination of Mitochondrial Membrane Potential (MMP)
4.7. Transwell Assays
4.8. Statistical Analysis
4.9. In Silico Prediction of the Antiproliferative Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.; Virós, A. Mechanisms of Drug Resistance in Melanoma. Handb. Exp. Pharmacol. 2018, 249, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.; Silveira, D. Natural Cytotoxic Diterpenoids, a Potential Source of Drug Leads for Melanoma Therapy. Curr. Pharm. Des. 2018, 24, 4237–4250. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C. Camptothecin and Taxol: Discovery to Clinic—Thirteenth Bruce F. Cain Memorial Award Lecture1. Cancer Res. 1995, 55, 753–760. [Google Scholar] [PubMed]
- González, M.A. Synthetic derivatives of aromatic abietane diterpenoids and their biological activities. Eur. J. Med. Chem. 2014, 87, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Cicconi, R.; Mignogna, G.; Giorgi, A.; Mattei, M.; Graziani, G.; Ferracane, R.; Grosso, A.; Aducci, P.; Schininà, M.E.; et al. Antiproliferative Effect of Rosmarinus officinalis L. Extract on Human Melanoma A375 Cells. PLoS ONE 2015, 10, e0132439. [Google Scholar] [CrossRef]
- Alcaraz, M.; Achel, D.G.; Olivares, A.; Olmos, E.; Alcaraz-Saura, M.; Castillo, J. Carnosol, radiation and melanoma: A translational possibility. Clin. Transl. Oncol. 2013, 15, 712–719. [Google Scholar] [CrossRef]
- Silva, C.O.; Molpeceres, J.; Batanero, B.; Fernandes, A.S.; Saraiva, N.; Costa, J.G.; Rijo, P.; Figueiredo, I.V.; Faísca, P.; Reis, C.P. Functionalized diterpene parvifloron D-loaded hybrid nanoparticles for targeted delivery in melanoma therapy. Ther. Deliv. 2016, 7, 521–544. [Google Scholar] [CrossRef]
- Habtemariam, S.; Varghese, G.K. A novel diterpene skeleton: Identification of a highly aromatic, cytotoxic and antioxidant 5-methyl-10-demethyl-abietane-type diterpene from Premna serratifolia. Phytother. Res. 2015, 29, 80–85. [Google Scholar] [CrossRef]
- Fronza, M.; Murillo, R.; Ślusarczyk, S.; Adams, M.; Hamburger, M.; Heinzmann, B.; Laufer, S.; Merfort, I. In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg. Med. Chem. 2011, 19, 4876–4881. [Google Scholar] [CrossRef]
- Faustino, C.; Neto, Í.; Fonte, P.; Macedo, A. Cytotoxicity and Chemotherapeutic Potential of Natural Rosin Abietane Diterpenoids and their Synthetic Derivatives. Curr. Pharm. Des. 2018, 24, 4362–4375. [Google Scholar] [CrossRef] [PubMed]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Ferruginol and Sugiol: A Short Review of their Chemistry, Sources, Contents, Pharmacological Properties and Patents. Trop. J. Nat. Prod. Res. TJNPR 2023, 7, 2325–2336. [Google Scholar] [CrossRef]
- Ho, S.T.; Tung, Y.T.; Kuo, Y.H.; Lin, C.C.; Wu, J.H. Ferruginol inhibits non-small cell lung cancer growth by inducing caspase-associated apoptosis. Integr. Cancer Ther. 2015, 14, 86–97. [Google Scholar] [CrossRef]
- Jung, S.N.; Shin, D.S.; Kim, H.N.; Jeon, Y.J.; Yun, J.; Lee, Y.J.; Kang, J.S.; Han, D.C.; Kwon, B.M. Sugiol inhibits STAT3 activity via regulation of transketolase and ROS-mediated ERK activation in DU145 prostate carcinoma cells. Biochem. Pharmacol. 2015, 97, 38–50. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Zhang, B.; Zhang, Y.; Li, J. Ferruginol induced apoptosis on SK-Mel-28 human malignant melanoma cells mediated through P-p38 and NF-κB. Human Exp. Toxicol. 2019, 38, 227–238. [Google Scholar] [CrossRef]
- González-Cardenete, M.A.; González-Zapata, N.; Boyd, L.; Rivas, F. Discovery of Novel Bioactive Tanshinones and Carnosol Analogues against Breast Cancer. Cancers 2023, 15, 1318. [Google Scholar] [CrossRef]
- González-Cardenete, M.A.; Hamulić, D.; Miquel-Leal, F.J.; González-Zapata, N.; Jimenez-Jarava, O.J.; Brand, Y.M.; Restrepo-Mendez, L.C.; Martinez-Gutierrez, M.; Betancur-Galvis, L.A.; Marín, M.L. Antiviral Profiling of C-18- or C-19-Functionalized Semisynthetic Abietane Diterpenoids. J. Nat. Prod. 2022, 85, 2044–2051. [Google Scholar] [CrossRef]
- Hamulić, D.; Stadler, M.; Hering, S.; Padrón, J.M.; Bassett, R.; Rivas, F.; Loza-Mejía, M.A.; Dea-Ayuela, M.A.; González-Cardenete, M.A. Synthesis and Biological Studies of (+)-Liquiditerpenoic Acid A (Abietopinoic Acid) and Representative Analogues: SAR Studies. J. Nat. Prod. 2019, 82, 823–831. [Google Scholar] [CrossRef]
- Martorana, A.; La Monica, G.; Bono, A.; Mannino, S.; Buscemi, S.; Palumbo Piccionello, A.; Gentile, C.; Lauria, A.; Peri, D. Antiproliferative Activity Predictor: A New Reliable In Silico Tool for Drug Response Prediction against NCI60 Panel. Int. J. Mol. Sci. 2022, 23, 14374. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Duarte, D.; Nunes, M.; Ricardo, S.; Vale, N. Combination of Antimalarial and CNS Drugs with Antineoplastic Agents in MCF-7 Breast and HT-29 Colon Cancer Cells: Biosafety Evaluation and Mechanism of Action. Biomolecules 2022, 12, 1490. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψm) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Savitskaya, M.A.; Onishchenko, G.E. Mechanisms of Apoptosis. Biochemistry 2015, 80, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef]
- Fackler, O.T.; Grosse, R. Cell motility through plasma membrane blebbing. J. Cell Biol. 2008, 181, 879–884. [Google Scholar] [CrossRef]
- Limame, R.; Wouters, A.; Pauwels, B.; Fransen, E.; Peeters, M.; Lardon, F.; De Wever, O.; Pauwels, P. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS ONE 2012, 7, e46536. [Google Scholar] [CrossRef]
- Gopal, S.; Kwon, S.J.; Ku, B.; Lee, D.W.; Kim, J.; Dordick, J.S. 3D tumor spheroid microarray for high-throughput, high-content natural killer cell-mediated cytotoxicity. Commun. Biol. 2021, 4, 893. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G. Common pitfalls in preclinical cancer target validation. Nat. Rev. Cancer 2017, 17, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Anseth, K.S. Recent advances in 3D models of tumor invasion. Curr. Opin. Biomed. Eng. 2021, 19, 100310. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Rahman, Z.; ten Dijke, P.; Boukany, P.E. Microfluidics meets 3D cancer cell migration. Trends Cancer 2022, 8, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Broccatelli, F.; Aliagas, I.; Zheng, H. Why Decreasing Lipophilicity Alone Is Often Not a Reliable Strategy for Extending IV Half-life. ACS Med. Chem. Lett. 2018, 9, 522–527. [Google Scholar] [CrossRef]
- Düssmann, H.; Rehm, M.; Kögel, D.; Prehn, J.H. Outer mitochondrial membrane permeabilization during apoptosis triggers caspase-independent mitochondrial and caspase-dependent plasma membrane potential depolarization: A single-cell analysis. J. Cell Sci. 2003, 116, 525–536. [Google Scholar] [CrossRef]
- Zamzami, N.; Kroemer, G. Methods to measure membrane potential and permeability transition in the mitochondria during apoptosis. Methods Mol. Biol. 2004, 282, 103–115. [Google Scholar] [CrossRef]
- Burmistrova, O.; Simões, M.F.T.; Rijo, P.C.; Quintana, J.; Bermejo, J.; Estévez, F. Antiproliferative Activity of Abietane Diterpenoids against Human Tumor Cells. J. Nat. Prod. 2013, 76, 1413–1423. [Google Scholar] [CrossRef]
- Prieto, J.M.; Hanafi, M.M.M. Advances in Molecular Regulation of Prostate Cancer Cells by Top Natural Products of Malaysia. Curr. Issues Mol. Biol. 2023, 45, 1536–1567. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Yang, S.F.; Hsieh, Y.H.; Hung, C.H.; Chu, S.C.; Yang, S.H.; Chen, P.N. The Inhibitory Effect of Abietic Acid on Melanoma Cancer Metastasis and Invasiveness In Vitro and In Vivo. Am. J. Chin. Med. 2015, 43, 1697–1714. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Li, X.; Liu, B.; Liu, Z. Mechanisms of Tanshinone II a inhibits malignant melanoma development through blocking autophagy signal transduction in A375 cell. BMC Cancer 2017, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter Six—Validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]
- Gowda, R.; Madhunapantula, S.V.; Kuzu, O.F.; Sharma, A.; Robertson, G.P. Targeting multiple key signaling pathways in melanoma using leelamine. Mol. Cancer Ther. 2014, 13, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Gowda, R.; Sharma, A.; Robertson, G.P. Leelamine mediates cancer cell death through inhibition of intracellular cholesterol transport. Mol. Cancer Ther. 2014, 13, 1690–1703. [Google Scholar] [CrossRef]
- Gowda, R.; Inamdar, G.S.; Kuzu, O.; Dinavahi, S.S.; Krzeminski, J.; Battu, M.B.; Voleti, S.R.; Amin, S.; Robertson, G.P. Identifying the structure-activity relationship of leelamine necessary for inhibiting intracellular cholesterol transport. Oncotarget 2017, 8, 28260–28277. [Google Scholar] [CrossRef]
- González, M.A.; Pérez-Guaita, D. Short syntheses of (+)-ferruginol from (+)-dehydroabietylamine. Tetrahedron 2012, 68, 9612–9615. [Google Scholar] [CrossRef]
- Robertson, G.P.; Raghavendragowda, C.D.; Madhunapantula, S.V.; Kuzu, O.F.; Inamdar, G.S. Compositions and Methods Relating to Proliferative Diseases. U.S. Patent 8,785,502, 31 July 2017. [Google Scholar]
- Lauria, A.; Mannino, S.; Gentile, C.; Mannino, G.; Martorana, A.; Peri, D. DRUDIT: Web-based DRUgs DIscovery Tools to design small molecules as modulators of biological targets. Bioinformatics 2020, 36, 1562–1569. [Google Scholar] [CrossRef]









| Compound | SK-MEL-28 | Fibroblasts | SI |
|---|---|---|---|
| 1 | 47.5 µM | 55 µM a | ≅1.2 |
| 2 | 9.8 µM | 75 µM a | ≅7.7 |
| Paclitaxel | 10 nM | 2.61 nM b | ≅0.3 |
| Compound | log P | MW | n-HBA | n-HBD | TPSA | Lipinski’s Violation |
|---|---|---|---|---|---|---|
| 1 | 6.41 | 286.46 | 1 | 1 | 20.23 | 1 |
| 2 | 4.67 | 301.47 | 2 | 3 | 46.25 | 0 |
| 3 | 5.29 | 372.50 | 4 | 0 | 52.61 | 1 |
| 4 | 5.73 | 330.47 | 3 | 1 | 46.53 | 1 |
| 5 | 4.39 | 386.49 | 5 | 0 | 69.68 | 0 |
| 6 | 5.11 | 316.44 | 3 | 2 | 57.53 | 1 |
| 7 | 5.24 | 302.46 | 2 | 2 | 40.46 | 1 |
| 8 | 4.83 | 344.45 | 4 | 1 | 63.60 | 0 |
| 9 | 4.21 | 330.42 | 4 | 2 | 74.60 | 0 |
| 10 | 4.33 | 316.44 | 3 | 2 | 57.53 | 0 |
| 11 | 5.51 | 300.44 | 2 | 1 | 37.30 | 1 |
| Rule of five | not > 5 | <500 | not > 10 | not > 5 | 1 violation allowed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, L.; González-Cardenete, M.A.; Prieto-Garcia, J.M. In Vitro Cytotoxic Effects of Ferruginol Analogues in Sk-MEL28 Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 16322. https://doi.org/10.3390/ijms242216322
Shao L, González-Cardenete MA, Prieto-Garcia JM. In Vitro Cytotoxic Effects of Ferruginol Analogues in Sk-MEL28 Human Melanoma Cells. International Journal of Molecular Sciences. 2023; 24(22):16322. https://doi.org/10.3390/ijms242216322
Chicago/Turabian StyleShao, Luying, Miguel A. González-Cardenete, and Jose M. Prieto-Garcia. 2023. "In Vitro Cytotoxic Effects of Ferruginol Analogues in Sk-MEL28 Human Melanoma Cells" International Journal of Molecular Sciences 24, no. 22: 16322. https://doi.org/10.3390/ijms242216322