Optimization of a Nucleophilic Two-Step Radiosynthesis of 6-O-(2-[18F]fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) for PET Imaging of Brain Opioid Receptors
Abstract
:1. Introduction
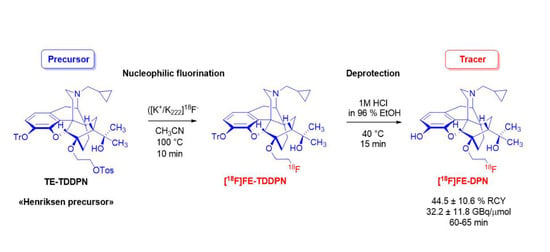
2. Results and Discussion
3. Conclusions
4. Methods and Materials
4.1. [18F]fluoride Production and Separation
4.2. Radiosynthesis of [18F]FE-DPN ([18F]11)
4.3. Purification of [18F]FE-DPN ([18F]11)
4.4. Quality Control
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benyhe, S.; Zádor, F.; Ötvös, F. Biochemistry of opioid (morphine) receptors: Binding, structure and molecular modelling. Acta Biol. Szeged 2015, 59 (Suppl. S1), 17–37. [Google Scholar]
- Stein, C. Opioid receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef]
- Cumming, P.; Marton, J.; Lilius, T.O.; Olberg, D.E.; Rominger, A. A Survey of molecular imaging of opioid receptors. Molecules 2019, 24, 4190. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous opiates and behavior: 2014. Peptides 2016, 75, 18–70. [Google Scholar] [CrossRef]
- Szűcs, E.; Büki, A.; Kékesi, G.; Horváth, G.; Benyhe, S. Mu-Opioid (MOP) receptor mediated G-protein signaling is impaired in specific brain regions in a rat model of schizophrenia. Neurosci. Lett. 2016, 619, 29–33. [Google Scholar] [CrossRef]
- Cumming, P.; Abi-Dargham, A.; Gründer, G. Molecular imaging of schizophrenia: Neurochemical findings in a heterogeneous and evolving disorder. Behav. Brain Res. 2021, 398, 113004. [Google Scholar] [CrossRef]
- Lever, J.R. PET and SPECT Imaging of the opioid system: Receptors, radioligands and avenues for drug discovery and development. Curr. Pharm. Des. 2007, 13, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, G.; Willoch, F. Imaging of opioid receptors in the central nervous system. Brain 2008, 131, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Dannals, R.F. Positron emission tomography radioligands for the opioid system. J. Label. Compd. Radiopharm. 2013, 56, 187–195. [Google Scholar] [CrossRef]
- Dannals, R.F.; Ravert, H.T.; Frost, J.J.; Wilson, A.A.; Burns, H.D.; Wagner, H.N. Radiosynthesis of an opiate receptor binding radiotracer: [11C]carfentanil. Int. J. Appl. Radiat. Isot. 1985, 36, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.J.; Wagner, H.N., Jr.; Dannals, R.F.; Ravert, H.T.; Links, J.M.; Wilson, A.A.; Burns, H.D.; Wong, D.F.; McPherson, R.W.; Rosenbaum, A.E.; et al. Imaging opiate receptors in the human brain by positron tomography. J. Comput. Assist. Tomogr. 1985, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.R.; Kinter, C.M.; Ravert, H.T.; Musachio, J.L.; Mathews, W.B.; Dannals, R.F. Synthesis of N1′-([11C]methyl)naltrindole ([11C]MeNTI): A radioligand for positron emission tomographic studies of delta opioid receptors. J. Label. Compd. Radiopharm. 1995, 36, 137–145. [Google Scholar] [CrossRef]
- Mathews, W.B.; Kinter, C.M.; Palma, J.; Daniels, R.V.; Ravert, H.T.; Dannals, R.F.; Lever, J.R. Synthesis of N1′-([18F]fluoroethyl)naltrindole ([18F]FEtNTI): A radioligand for positron emission tomographic studies of delta opioid receptors. J. Label. Compd. Radiopharm. 1999, 42, 43–54. [Google Scholar] [CrossRef]
- Naylor, A.; Judd, D.B.; Lloyd, J.E.; Scopes, D.I.C.; Hayes, A.G.; Birch, P.J. A potent new class of kappa-receptor agonist: 4-substituted 1-(arylacetyl)-2-[(dialkylamino)methyl]piperazines. J. Med. Chem. 1993, 36, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Ravert, H.T.; Mathews, W.B.; Musachio, J.L.; Scheffel, U.; Finley, P.; Dannals, R.F. [11C]-methyl 4-[(3, 4-dichlorophenyl) acetyl]-3-[(1-pyrrolidinyl) methyl]-1- piperazinecarboxylate ([11C] GR89696): Synthesis and in vivo binding to kappa opiate receptors. Nucl. Med. Biol. 1999, 26, 737–741. [Google Scholar] [CrossRef]
- Schoultz, B.W.; Arstad, E.; Marton, J.; Willoch, F.; Drzezga, A.; Wester, H.J.; Henriksen, G. A new method for radiosynthesis of 11C-labeled carbamate groups and its application for a highly efficient synthesis of the kappa-opioid receptor tracer [11C]GR103545. Open Med. Chem. J. 2008, 2, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, B.W.; Hjornevik, T.; Willoch, F.; Marton, J.; Noda, A.; Murakami, Y.; Miyoshi, S.; Nishimura, S.; Årstad, E.; Drzezga, A.; et al. Evaluation of the kappa-opioid receptor-selective tracer [11C]GR103545 in awake rhesus macaques. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1174–1180. [Google Scholar] [CrossRef]
- Naganawa, M.; Jacobsen, L.K.; Zheng, M.-Q.; Lin, S.-F.; Banerjee, A.; Byon, W.; Weinzimmer, D.; Tomasi, G.; Nabulsi, N.; Grimwood, S.; et al. Evaluation of the agonist PET radioligand [11C]GR103545 to image kappa opioid receptor in humans: Kinetic model selection, test-retest reproducibility and receptor occupancy by the antagonist PF-04455242. NeuroImage 2014, 99, 69–79. [Google Scholar] [CrossRef]
- Li, S.; Zheng, M.-Q.; Naganawa, M.; Kim, S.; Gao, H.; Kapinos, M.; Labaree, D.; Huang, Y. Development and In Vivo Evaluation of a κ-Opioid Receptor Agonist as a PET Radiotracer with Superior Imaging Characteristics. J. Nucl. Med. 2019, 60, 1023–1030. [Google Scholar] [CrossRef]
- Li, S.; Zheng, M.-Q.; Naganawa, M.; Gao, H.; Pracitto, R.; Shirali, A.; Lin, S.-F.; Teng, J.-K.; Ropchan, J.; Huang, Y. Novel kappa opioid receptor agonist as improved PET radiotracer: Development and in vivo evaluation. Mol. Pharm. 2019, 16, 1523–1531. [Google Scholar] [CrossRef]
- Li, S.; Cai, Z.; Zheng, M.-Q.; Holden, D.; Naganawa, M.; Lin, S.-F.; Ropchan, J.; Labaree, D.; Kapinos, M.; Lara-Jaime, T.; et al. Novel 18F-labeled κ-opioid receptor antagonist as PET radiotracer: Synthesis and in vivo evaluation of 18F-LY2459989 in nonhuman primates. J. Nucl. Med. 2018, 59, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Pike, V.W.; Rash, K.S.; Chen, Z.; Pedregal, C.; Statnick, M.A.; Kimura, Y.; Hong, J.; Zoghbi, S.S.; Fujita, M.; Toledo, M.A.; et al. Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J. Med. Chem. 2011, 54, 2687–2700. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, E.D.; Sanabria-Bohórquez, S.; Eng, W.; Joshi, A.D.; Patel, S.; Gibson, R.E.; O'Malley, S.; Krause, S.M.; Ryan, C.; Riffel, K.; et al. Evaluation of [18F]MK-0911, a positron emission tomography (PET) tracer for opioid receptor-like 1 (ORL1), in rhesus monkey and human. NeuroImage 2013, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rothman, R.B.; Bykov, V.; Reid, A.; De Costa, B.R.; Newman, A.H.; Jacobson, A.E.; Rice, K.C. A brief study of the selectivity of norbinaltorphimine, (-)-cyclofoxy, and (+)-cyclofoxy among opioid receptor subtypes in vitro. Neuropeptides 1988, 12, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Channing, M.; Finn, R.; Dunn, B.B.; Simpson, N.; Carson, R.; Ostrowski, N.; Cohen, R.; Burke, T.; Larson, S.; et al. Ligands for Imaging Opioid Receptors in Conscious Humans by Positron Emission Tomography (PET). NIDA Res. Monogr. 1988, 90, 117–121. [Google Scholar]
- Wester, H.-J.; Willoch, F.; Tölle, T.R.; Munz, F.; Herz, M.; Øye, I.; Schadrack, J.; Schwaiger, M.; Bartenstein, P. 6-O-(2-[18F]Fluoroethyl-6-O-desmethyldiprenorphine ([18F]DPN): Synthesis, biologic evaluation, and comparison with [11C]DPN in humans. J. Nucl. Med. 2000, 41, 1279–1286. [Google Scholar]
- Bentley, K.W.; Hardy, D.G. Novel analgesics and molecular rearrangements in the morphine-thebaine group I. Ketones derived from 6,14-endo-ethenotetrahydrothebaine. J. Am. Chem. Soc. 1967, 89, 3267–3273. [Google Scholar]
- Bentley, K.W.; Hardy, D.G.; Meek, B. Novel analgesics and molecular rearrangements in the morphine-thebaine group. II. Alcohols derived from 6,14-endo-etheno- and 6,14-endo-ethanotetrahydrothebaine. J. Am. Chem. Soc. 1967, 89, 3273–3280. [Google Scholar] [CrossRef]
- Bentley, K.W.; Hardy, D.G. Novel analgesics and molecular rearrangements in the morphine-thebaine group. III. Alcohols of the 6,14-endo-ethenotetrahydrooripavine series and derived analogs of N-allylnormorphine and -norcodeine. J. Am. Chem. Soc. 1967, 89, 3281–3292. [Google Scholar] [CrossRef]
- Blane, G.F. Blockade of bradykinin-induced nociception in the rat as a test for analgesic drugs with particular reference to morphine antagonists. J. Pharm. Pharmacol. 1967, 19, 367–373. [Google Scholar] [CrossRef]
- Casy, A.F.; Parfitt, R.T. Diels-Alder adducts of thebaine. In Opioid Analgesics. Chemistry and Receptors; Plenum Press: New York, NY, USA, 1986; pp. 69–84. [Google Scholar]
- Jessup, D.A.; Clark, W.E.; Jones, K.R.; Clark, R.; Lance, W.R. Immobilization of free-ranging desert bighorn sheep, tule elk, and wild horses, using carfentanil and xylazine: Reversal with naloxone, diprenorphine, and yohimbine. J. Am. Vet. Med. Assoc. 1985, 187, 1253–1254. [Google Scholar] [PubMed]
- Herz, A.; Höllt, V. Receptor occupation and pharmacological activity as demonstrated on opiates. Arzneim.-Forsch. / Drug. Res. 1977, 27, 1865–1867. [Google Scholar]
- Frost, J.J.; Smith, A.C.; Wagner, H.N. [3H]-diprenorphine is selective for mu opiate receptors in vivo. Life Sci. 1986, 38, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.P.; Luthra, S.K.; Maziere, B.; Pike, V.W.; Loch, C.; Crouzel, C.; Syrota, A.; Jones, T. Regional cerebral opioid receptor studies with [11C]diprenorphine in normal volunteers. J. Neurosci. Methods 1988, 23, 121–129. [Google Scholar] [CrossRef]
- Raynor, K.; Kong, H.; Chen, Y.; Yasuda, K.; Yu, L.; Bell, G.I.; Reisine, T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol. Pharmacol. 1994, 45, 330–334. [Google Scholar] [PubMed]
- Levinstein, M.R.; Ventriglia, E.N.; Gomez, J.L.; Budinich, R.C.; Marton, J.; Henriksen, G.; Holt, D.P.; Dannals, R.F.; Pomper, M.G.; Zarate, C.A.; et al. 6-O-(2-[18F]Fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) preferentially binds to mu opioid receptors in vivo. Mol. Imaging Biol. 2023, 25, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Luthra, S.K.; Pike, V.W.; Brady, F. The preparation of carbon-11 labelled diprenorphine: A new radioligand for the study of the opiate receptor system in vivo. J. Chem. Soc. Chem. Commun. 1985, 1423–1425. [Google Scholar] [CrossRef]
- Kopcho, J.J.; Schaeffer, J.C. Selective O-demethylation of 7.alpha.-(aminomethyl)-6,14-endo-ethenotetrahydrothebaine. J. Org. Chem. 1986, 51, 1620–1622. [Google Scholar] [CrossRef]
- Lever, J.R.; Dannals, R.F.; Wilson, A.A.; Ravert, H.T.; Wagner, H.N., Jr. Synthesis of carbon-11 labeled diprenorphine: A radioligand for positron emission tomographic studies of opiate receptors. Tetrahedron Lett. 1987, 28, 4015–4018. [Google Scholar] [CrossRef]
- Luthra, S.K.; Brady, F.; Turton, D.R.; Brown, D.J.; Dowsett, K.; Waters, S.L.; Jones, A.K.P.; Matthews, R.W.; Crowder, J.C. Automated radiosyntheses of [6-O-methyl-11C]diprenorphine and [6-O-methyl-11C]buprenorphine from 3-O-trityl protected precursors. Appl. Radiat. Isot. 1994, 45, 857–873. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 radiochemistry, labeling strategies and synthetic routes. Bioconjugate Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Guan, B.; Zhou, N.; Wu, C.-Y.; Li, S.; Chen, Y.-A.; Debnath, S.; Hofstad, M.; Ma, S.; Raj, G.V.; He, D.; et al. Validation of SV2A-Targeted PET Imaging for Noninvasive Assessment of Neuroendocrine Differentiation in Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 13085. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Hao, G.; Guan, B.; Thapa, P.; Hao, J.; Hammers, H.; Sun, X. Theranostic Small-Molecule Prodrug Conjugates for Targeted Delivery and Controlled Release of Toll-like Receptor 7 Agonists. Int. J. Mol. Sci. 2022, 23, 7160. [Google Scholar] [CrossRef]
- Debnath, S.; Zhou, N.; McLaughlin, M.; Rice, S.; Pillai, A.K.; Hao, G.; Sun, X. PSMA-Targeting Imaging and Theranostic Agents— Current Status and Future Perspective. Int. J. Mol. Sci. 2022, 23, 1158. [Google Scholar] [CrossRef]
- Schoultz, B.W.; Reed, B.J.; Marton, J.; Willoch, F.; Henriksen, G. A fully automated radiosynthesis of [18F]fluoroethyl-diprenorphine on a single module by use of SPE cartridges for preparation of high quality 2-[18F]fluoroethyl tosylate. Molecules 2013, 18, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, B.W.; Hjørnevik, T.; Reed, B.J.; Marton, J.; Coello, C.S.; Willoch, F.; Henriksen, G. Synthesis and evaluation of three structurally related 18F-labeled orvinols of different intrinsic activities: 6-O-[18F]fluoroethyl-diprenorphine ([18F]FDPN), 6-O-[18F]fluoroethyl-buprenorphine ([18F]FBPN) and 6-O-[18F]fluoroethyl-phenethyl-orvinol ([18F]FPEO). J. Med. Chem. 2014, 57, 5464–5469. [Google Scholar]
- Spilker, M.E.; Sprenger, T.; Valet, M.; Henriksen, G.; Wagner, K.; Wester, H.-J.; Toelle, T.R.; Boecker, H. Quantification of [18F]diprenorphine kinetics in the human brain with compartmental and non-compartmental modeling approaches. NeuroImage 2004, 22, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Boecker, H.; Sprenger, T.; Henriksen, G.; Toelle, T.R.; Spilker, M.E. Optimal duration of PET studies with 18F-fluoroethyl-diprenorphine. J. Nucl. Med. 2005, 46, 2092–2096. [Google Scholar] [PubMed]
- Henriksen, G.; Spilker, M.E.; Sprenger, T.; Hauser, A.I.; Platzer, S.; Boecker, H.; Toelle, T.R.; Wester, H.J. Gender dependent rate of metabolism of the opioid receptor-PET ligand [18F]fluoroethyldiprenorphine. Nuklearmedizin 2006, 45, 197–200. [Google Scholar]
- Baumgärtner, U.; Buchholz, H.-G.; Bellosevich, A.; Magerl, W.; Siessmeier, T.; Rolke, R.; Höhnemann, S.; Piel, M.; Rösch, F.; Wester, H.-J.; et al. High opiate receptor binding potential in the human lateral pain system. NeuroImage 2006, 30, 692–699. [Google Scholar] [CrossRef]
- Sprenger, T.; Valet, M.; Boecker, H.; Henriksen, G.; Spilker, M.E.; Willoch, F.; Wagner, K.J.; Wester, H.-J.; Toelle, T.R. Opioidergic activation in the medial pain system after heat pain. Pain 2006, 122, 63–67. [Google Scholar] [CrossRef]
- Sprenger, T.; Henriksen, G.; Platzer, S.; Berthele, A.; Tölle, T.R. Positronenemissions-tomographie (PET) in der Schmerzforschung. Von der Struktur zur Aktivität des Opiatrezeptorsystems. Schmerz 2007, 21, 503–513. [Google Scholar] [CrossRef]
- Boecker, H.; Henriksen, G.; Sprenger, T.; Miederer, I.; Willoch, F.; Valet, M.; Berthele, A.; Tölle, T.R. Positron emission tomography ligand activation studies in the sport sciences: Measuing neurochemistry in vivo. Methods 2008, 45, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Boecker, H.; Sprenger, T.; Spilker, M.E.; Henriksen, G.; Koppenhoefer, M.; Wagner, K.J.; Valet, M.; Berthele, A.; Toelle, T.R. The Runner's High: Opioidergic Mechanisms in the Human Brain. Cereb. Cortex 2008, 18, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberger, M.; Klega, A.; Gründer, G.; Buchholz, H.-G.; Scheurich, A.; Schirrmacher, R.; Schirrmacher, E.; Müller, C.; Henriksen, G.; Bartenstein, P. Opioid receptor PET reveals the psychobiologic correlates of reward processing. J. Nucl. Med. 2008, 49, 1257–1261. [Google Scholar] [CrossRef]
- Mueller, C.; Klega, A.; Buchholz, H.-G.; Rolke, R.; Magerl, W.; Schirrmacher, R.; Schirrmacher, E.; Birklein, F.; Treede, R.-D.; Schreckenberger, M. Basal opioid receptor binding is associated with differences in sensory perception in healthy human subjects: A [18F]diprenorphine PET study. NeuroImage 2010, 49, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.J.; Pitcher, M.H.; Stone, L.S.; Tarum, F.; Niu, G.; Chen, X.; Kiesewetter, D.O.; Schweinhardt, P.; Bushnell, M.C. Chronic neuropathic pain reduces opioid receptor availability with associated anhedonia in rat. Pain 2018, 159, 1856–1866. [Google Scholar] [CrossRef]
- Marton, J.; Cumming, P.; Bauer, B.; Henriksen, G. A new precursor for the radiosynthesis of 6-O-(2-[18F]fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) by nucleophilic radiofluorination. Lett. Org. Chem. 2021, 18, 344–352. [Google Scholar] [CrossRef]
- Marton, J.; Sipos, A.; Henriksen, G.; Cumming, P.; Berényi, S.; Schmitt, B.M.; Szabó, Z. NMR Analysis of a series of 6,14-ethenomorphinan derivatives as PET precursors and reference substances. ChemistrySelect 2021, 6, 5994–6005. [Google Scholar] [CrossRef]
- Racz, L. Comparison of phase transfer catalysts for labelling with 18F isotope. BSc Thesis, University of Debrecen, Debrecen, Hungary, 2013. [Google Scholar]
- Song, R.; Tago, T.; Tatsuta, M.; Shiraishi, N.; Iwai, K.; Hirano, K.; Toyohara, J.; Tanaka, H. N-Alkyl 3-aminobut-2-enenitrile as a Non-radioactive Side Product in Nucleophilic 18F-Fluorination. ChemistrySelect 2021, 6, 2826–2831. [Google Scholar] [CrossRef]









| Cartridge | Eluent Composition | Bound [18F]F- (%) | Eluted [18F]F- (%) | [18F]F- Recovery (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Base (mg) | Kryptofix 222 (mg) | Water (µL) | Acetonitrile (µL) | ||||||
| K2CO3 (mg) | KHCO3 (mg) | Bu4NHCO3 (mg) | |||||||
| Sep-Pak QMA Plus Light | 1.68 | - | - | 10 | 120 | 2000 | 99 ± 1 | 97.2 ± 2.1 | 96.2 ± 1.9 |
| - | 1.2 | - | 5.5 | 120 | 500 | 99 | 50.5 | 50.0 | |
| - | 1.8 | - | 9 | 180 | 2000 | 100 | 97.4 | 97.4 | |
| SOLA-AX SPE (10 mg) | 0.35 | - | - | 1 | 47 | 500 | 48 ± 27 | 96.8 ± 1.7 | 46.5 ± 25.3 |
| Oasis Max 1cc (10 mg) | 0.35 | - | - | 1 | 47 | 500 | 97.6 ± 1.6 | 92.0 ± 3.7 | 89.8 ± 4.9 |
| - | - | 0.72 | - | 50 | 500 | 99.4 ± 0.3 | 94.1 ± 1.0 | 93.5 ± 0.9 | |
| Cartridge | K+ (µmol) | Precursor (µmol) | K+/Precursor Molar Ratio | Radiochemical Yield ** Corrected with Elution Efficiency (%) | Molar Activity (GBq/µmol) |
|---|---|---|---|---|---|
| QMA light SepPak | 24.31 | 1.17 | 20.8 | <1 (n = 2) | n.d. |
| 17.98 | 2.35 | 7.7 | 6.9 (n = 1) | 94 | |
| 11.99 | 2.35 | 5.1 | 6.3 (n = 1) | 4.4 | |
| OasisMax 1cc | 5.06 | 2.35 | 2.2 | 44.5 ± 10.6 (n = 3) | 32.2 ± 11.8 |
| 3.75 * | 2.35 | 1.6 * | 40.8 ± 30.3 (n = 2) | 20.4 ± 21.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, E.; Gyuricza, B.; Forgács, V.; Cumming, P.; Henriksen, G.; Marton, J.; Bauer, B.; Mikecz, P.; Fekete, A. Optimization of a Nucleophilic Two-Step Radiosynthesis of 6-O-(2-[18F]fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) for PET Imaging of Brain Opioid Receptors. Int. J. Mol. Sci. 2023, 24, 13152. https://doi.org/10.3390/ijms241713152
Németh E, Gyuricza B, Forgács V, Cumming P, Henriksen G, Marton J, Bauer B, Mikecz P, Fekete A. Optimization of a Nucleophilic Two-Step Radiosynthesis of 6-O-(2-[18F]fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) for PET Imaging of Brain Opioid Receptors. International Journal of Molecular Sciences. 2023; 24(17):13152. https://doi.org/10.3390/ijms241713152
Chicago/Turabian StyleNémeth, Enikő, Barbara Gyuricza, Viktória Forgács, Paul Cumming, Gjermund Henriksen, János Marton, Beate Bauer, Pál Mikecz, and Anikó Fekete. 2023. "Optimization of a Nucleophilic Two-Step Radiosynthesis of 6-O-(2-[18F]fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) for PET Imaging of Brain Opioid Receptors" International Journal of Molecular Sciences 24, no. 17: 13152. https://doi.org/10.3390/ijms241713152