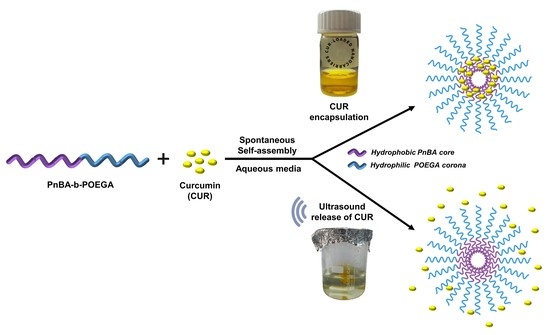
Curcumin-Loaded PnBA-b-POEGA Nanoformulations: A Study of Drug-Polymer Interactions and Release Behavior
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of PnBA-b-POEGA Block Copolymers and Preparation of CUR-Loaded PnBA-b-POEGA Nanocarriers
2.2. Physicochemical Characterization of CUR-Loaded PnBA-b-POEGA Nanocarriers
2.3. 1H-NMR Studies on CUR-Loaded PnBA-b-POEGA Nanocarriers
2.4. 2D-COSY Studies on CUR-Loaded PnBA-b-POEGA Nanocarriers
2.5. 2D-NOESY Studies on CUR-Loaded PnBA-b-POEGA Nanocarriers
2.6. 2D-DOSY Studies on CUR-Loaded PnBA-b-POEGA Nanocarriers
2.7. 1H-NMR Stability Studies on CUR-Loaded PnBA-b-POEGA Nanocarriers
2.8. Encapsulation and Release Studies
3. Materials and Methods
3.1. Materials
3.2. Preparation of CUR-Loaded PnBA-b-POEGA Nanocarriers
3.3. Drug Loading and Encapsulation Efficiency Calculations of CUR
3.4. Release Studies
3.5. NMR Sample Preparation
3.6. Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. CMLS 2004, 61, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliver. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliver. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Han, G.; Ju, Y.; Zhao, H. Synthesis of amphiphilic block-type macromolecular brushes with cleavable pendant chains and fabrication of micelle-templated polymer nanocapsules. Polym. Chem. 2016, 7, 1197–1206. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Meleshko, T.K.; Kashina, A.V.; Yakimansky, A.V. Amphiphilic multicomponent molecular brushes. Russ. Chem. Rev. 2019, 88, 1248. [Google Scholar] [CrossRef]
- Ozer, I.; Tomak, A.; Zareie, H.M.; Baran, Y.; Bulmus, V. Effect of Molecular Architecture on Cell Interactions and Stealth Properties of PEG. Biomacromolecules 2017, 18, 2699–2710. [Google Scholar] [CrossRef]
- Hu, X.; Jing, X. Biodegradable amphiphilic polymer–drug conjugate micelles. Expert Opin. Drug Deliv. 2009, 6, 1079–1090. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Kumari, V.; Kumari, P.; Samanta, S. Amphiphilic block copolymers as potential drug delivery agent for curcumin: A review. Mater. Today Proc. 2021, 43, 2944–2948. [Google Scholar] [CrossRef]
- Sahu, A.; Bora, U.; Kasoju, N.; Goswami, P. Synthesis of novel biodegradable and self-assembling methoxy poly (ethylene glycol)–palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008, 4, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thong, P.Q.; Nam, N.H.; Phuc, N.X.; Manh, D.H.; Thu, H.P. Impact of PLA/PEG ratios on curcumin solubility and encapsulation efficiency, size and release behavior of curcumin loaded poly (lactide)-poly (ethylenglycol) polymeric micelles. Int. J. Drug Deliv. 2014, 6, 279–285. [Google Scholar]
- Liang, H.; Friedman, J.M.; Nacharaju, P. Fabrication of biodegradable PEG–PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Angarita, A.V.; Umaña-Perez, A.; Perez, L.D. Enhancing the performance of PEG-b-PCL-based nanocarriers for curcumin through its conjugation with lipophilic biomolecules. J. Bioact. Compat. Polym. 2020, 35, 399–413. [Google Scholar] [CrossRef]
- Ma, Z.; Haddadi, A.; Molavi, O.; Lavasanifar, A.; Lai, R.; Samuel, J. Micelles of poly (ethylene oxide)-b-poly (ε-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 86, 300–310. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J. Biomater. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef]
- Zhao, L.; Du, J.; Duan, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: Preparation, optimization and in vitro characterization. Colloids Surf. B 2012, 97, 101–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Rauf Khan, A.; Fu, M.; Zhai, Y.; Ji, J.; Bobrovskaya, L.; Zhai, G. Advances in curcumin-loaded nanopreparations: Improving bioavailability and overcoming inherent drawbacks. J. Drug Target. 2019, 27, 917–931. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 4449–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-guided brain drug delivery: Expanding the therapeutic approach to neurodegenerative diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Mague, J.T.; Alworth, W.L.; Payton, F.L. Curcumin and derivatives. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2004, 60, o608–o610. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, J.H.; Cho, H.Y.; Yum, Y.N.; Kim, S.H.; Park, H.-J.; Shim, B.S.; Choi, S.H.; Kwon, H.J. Irreversible inhibition of CD13/aminopeptidase N by the antiangiogenic agent curcumin. Chem. Biol. 2003, 10, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Wortelboer, H.M.; Usta, M.; van der Velde, A.E.; Boersma, M.G.; Spenkelink, B.; van Zanden, J.J.; Rietjens, I.M.; van Bladeren, P.J.; Cnubben, N.H. Interplay between MRP inhibition and metabolism of MRP inhibitors: The case of curcumin. Chem. Res. Toxicol. 2003, 16, 1642–1651. [Google Scholar] [CrossRef]
- Bong, P.H. Spectral and photophysical behaviors of curcumin and curcuminoids. Bull. Korean Chem. Soc. 2000, 21, 81–86. [Google Scholar]
- Pedersen, U.; Rasmussen, P.B.; Lawesson, S.O. Synthesis of naturally occurring curcuminoids and related compounds. Liebigs Ann. Chem. 1985, 1985, 1557–1569. [Google Scholar] [CrossRef]
- Fan, T.W.-M.; Lane, A.N. Applications of NMR spectroscopy to systems biochemistry. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 92, 18–53. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.C. Micellar systems: Nuclear magnetic resonance spectroscopy. Encycl. Surf. Colloid Sci. 2006, 5, 3742. [Google Scholar]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Nano-Assemblies from Amphiphilic PnBA-b-POEGA Copolymers as Drug Nanocarriers. Polymers 2021, 13, 1164. [Google Scholar] [CrossRef] [PubMed]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Biocompatible PEO-b-PCL nanosized micelles as drug carriers: Structure and drug–polymer interactions. Nanomaterials 2020, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Chroni, A.; Mavromoustakos, T.; Pispas, S. Poly (2-oxazoline)-Based Amphiphilic Gradient Copolymers as Nanocarriers for Losartan: Insights into Drug–Polymer Interactions. Macromol 2021, 1, 177–200. [Google Scholar] [CrossRef]
- Chroni, A.; Pispas, S.; Forys, A.; Trzebicka, B. pH-Driven Morphological Diversity in Poly [n-Butyl Acrylate-block-(2-(Dimethylamino) Ethyl Acrylate)] Amphiphilic Copolymer Solutions. Macromol. Rapid Commun. 2019, 40, 1900477. [Google Scholar] [CrossRef]
- Ismail, E.; Sabry, D.; Mahdy, H.; Khalil, M. Synthesis and Characterization of some Ternary Metal Complexes of Curcumin with 1, 10-phenanthroline and their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, S.; Natarajan, R.; Natarajan, S.; Rathikha, R. Structural investigation on curcumin. Asian J. Chem. 2008, 20, 2903. [Google Scholar]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [Green Version]
- Pandit, R.S.; Gaikwad, S.C.; Agarkar, G.A.; Gade, A.K.; Rai, M. Curcumin nanoparticles: Physico-chemical fabrication and its in vitro efficacy against human pathogens. 3 Biotech 2015, 5, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Prasad, C.; Bhatia, E.; Banerjee, R. Curcumin encapsulated lecithin nanoemulsions: An oral platform for ultrasound mediated spatiotemporal delivery of curcumin to the tumor. Sci. Rep. 2020, 10, 8587. [Google Scholar] [CrossRef]
- Fang, X.-B.; Zhang, J.-M.; Xie, X.; Liu, D.; He, C.-W.; Wan, J.-B.; Chen, M.-W. pH-sensitive micelles based on acid-labile pluronic F68–curcumin conjugates for improved tumor intracellular drug delivery. Int. J. Pharm. 2016, 502, 28–37. [Google Scholar] [CrossRef]
- Prasad, D.; Praveen, A.; Mahapatra, S.; Mogurampelly, S.; Chaudhari, S.R. Existence of β-diketone form of curcuminoids revealed by NMR spectroscopy. Food Chem. 2021, 360, 130000. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, C.; Zhang, Q.; Gao, M.; Zhang, J.; Kong, D.; Zhao, Y. Tuning the architecture of polymeric conjugate to mediate intracellular delivery of pleiotropic curcumin. Eur. J. Pharm. Biopharm. 2015, 90, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, M.; Chen, C.; Fan, A.; Zhang, J.; Kong, D.; Wang, Z.; Peer, D.; Zhao, Y. Triggered-release polymeric conjugate micelles for on-demand intracellular drug delivery. Nanotechnology 2015, 26, 115101. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, S.; Kong, D.; Gao, X.; Zhao, Y.; Wang, Z. Biodegradable polymer-curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm. Res. 2012, 29, 3512–3525. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Borden, M.A. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, N. Ultrasound-mediated micellar drug delivery. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group 2012, 28, 374–385. [Google Scholar] [CrossRef]
- Xia, H.; Zhao, Y.; Tong, R. Ultrasound-Mediated Polymeric Micelle Drug Delivery. Adv. Exp. Med. Biol. 2016, 880, 365–384. [Google Scholar] [CrossRef]
- Salgarella, A.R.; Zahoranova, A.; Sramkova, P.; Majercikova, M.; Pavlova, E.; Luxenhofer, R.; Kronek, J.; Lacik, I.; Ricotti, L. Investigation of drug release modulation from poly(2-oxazoline) micelles through ultrasound. Sci. Rep. 2018, 8, 9893. [Google Scholar] [CrossRef] [Green Version]
- Chroni, A.; Chrysostomou, V.; Skandalis, A.; Pispas, S. Drug Delivery: Hydrophobic Drug Encapsulation into Amphiphilic Block Copolymer Micelles. In Supramolecules in Drug Discovery and Drug Delivery; Springer: Berlin, Germany, 2021; pp. 71–83. [Google Scholar]
- Lübtow, M.M.; Lorson, T.; Finger, T.; Gröber-Becker, F.K.; Luxenhofer, R. Combining ultra-high drug-loaded micelles and injectable hydrogel drug depots for prolonged drug release. Macromol. Chem. Phys. 2020, 221, 1900341. [Google Scholar] [CrossRef] [Green Version]
- Pöppler, A.C.; Lübtow, M.M.; Schlauersbach, J.; Wiest, J.; Meinel, L.; Luxenhofer, R. Loading-Dependent Structural Model of Polymeric Micelles Encapsulating Curcumin by Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 18540–18546. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Teng, C.P.; Win, K.Y.; Chen, Y.; Zhang, X.; Yang, D.P.; Li, Z.; Ye, E. Polymeric encapsulation of turmeric extract for bioimaging and antimicrobial applications. Macromol. Rapid Commun. 2019, 40, 1800216. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Ha, P.T.; Sao Nguyen, A.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025001. [Google Scholar] [CrossRef]
















| Sample | Mw a (× 104 g mol−1) | wt. % PnBA b |
|---|---|---|
| PnBA30-b-POEGA70 | 1.29 | 30 |
| PnBA27-b-POEGA73 | 2.86 | 27 |
| Sample | Rh a (nm) | PDI a | Intensity a (a.u) |
|---|---|---|---|
| PnBA30-b-POEGA70 | 10 | 0.12 | 167 |
| PnBA30-b-POEGA70 + 20% CUR | 14 | 0.21 | 210 |
| PnBA30-b-POEGA70 + 50% CUR | 28 | 0.25 | 470 |
| PnBA27-b-POEGA73 | 24 | 0.15 | 960 |
| PnBA27-b-POEGA73 + 20% CUR | 22 | 0.16 | 926 |
| PnBA27-b-POEGA73 + 50% CUR | 35 | 0.31 | 4000 |
| 1H-NMR Chemical Shifts of PnBA30-b-POEGA70 Copolymer (ppm) | |||
|---|---|---|---|
| Protons of Polymer Structure |
|
|
|
| Ha-b | 2.13 | 2.14 | 2.14 |
| Hc | 3.94 | 4.05 | 4.05 |
| Hd | 1.51 | 1.52 | 1.52 |
| He | 1.1–1.34 | 1.1–1.31 | 1.09–1.32 |
| Hf | 0.86 | 0.86 | 0.86 |
| Hg | 4.17 | 4.19 | 4.18 |
| Hh | 3.60 | 3.62 | 3.62 |
| Hi | 3.28 | 3.31 | 3.30 |
| 1H-NMR Chemical Shifts (ppm) | ||
|---|---|---|
| Protons of CUR Structure |
|
|
| H1 | 5.70 | 5.87 |
| H22′ | 5.91–5.93 | 6.53 |
| H33′ | 6.36–6.40 | 7.62 |
| H44′-H77′-H88′ | 6.22–6.12–6.15 | 7.01–7.38 |
| H55′ | 3.63 | 4.72 |
| H66′ | 8.37 | - |
| Sample | DL% | EE% | % Max. Release Rate of CUR without Sonication | % Max. Release Rate of CUR with Sonication |
|---|---|---|---|---|
| PnBA30-b-POEGA70 + 50%CUR | 12 | 88 | 9 | 38 |
| PnBA27-b-POEGA73 + 20%CUR | 13 | 86 | 15 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chroni, A.; Mavromoustakos, T.; Pispas, S. Curcumin-Loaded PnBA-b-POEGA Nanoformulations: A Study of Drug-Polymer Interactions and Release Behavior. Int. J. Mol. Sci. 2023, 24, 4621. https://doi.org/10.3390/ijms24054621
Chroni A, Mavromoustakos T, Pispas S. Curcumin-Loaded PnBA-b-POEGA Nanoformulations: A Study of Drug-Polymer Interactions and Release Behavior. International Journal of Molecular Sciences. 2023; 24(5):4621. https://doi.org/10.3390/ijms24054621
Chicago/Turabian StyleChroni, Angeliki, Thomas Mavromoustakos, and Stergios Pispas. 2023. "Curcumin-Loaded PnBA-b-POEGA Nanoformulations: A Study of Drug-Polymer Interactions and Release Behavior" International Journal of Molecular Sciences 24, no. 5: 4621. https://doi.org/10.3390/ijms24054621