TZD-Based Hybrid Molecules Act as Dual Anti-Mycobacterium tuberculosis and Anti-Toxoplasma gondii Agents
Abstract
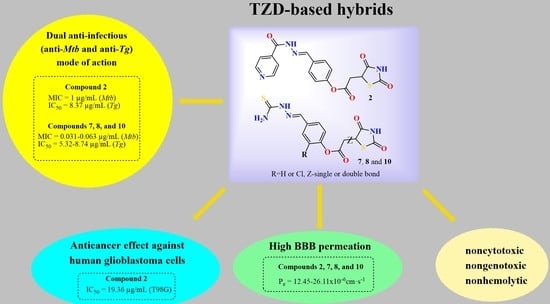
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Inhibition of T. gondii Tachyzoites In Vitro
2.3. In Vivo Toxicity Tests and Hemolytic Activity and Cytotoxic Effects on Host Cells
2.4. Cytotoxic Effect against Human Glioblastoma T98G Cells
2.5. Clinical Significance of the Findings
3. Materials and Methods
3.1. Chemistry
- General procedure for the synthesis of TZD-PCH (1–6) hybrid molecules
- General procedure for the synthesis of TZD-TSC (7–12) hybrid molecules
3.2. Compound and Drug Preparation
3.3. Cell and Parasite Culture
3.4. In Vitro Anti-Tg Assay
3.5. Cytotoxicity Assay
3.6. Danio Rerio Culture and Fish Embryo Toxicity Test (FET)
3.7. Hemolytic Activity Determination
3.8. PAMPA-BBB Assay
3.9. Cytotoxicity against Human Glioblastoma T98G Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petit-Jentreau, L.; Tailleux, L.; Coombes, J.L. Purinergic signaling: A common path in the macrophage response against Mycobacterium tuberculosis and Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2017, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 27 September 2022).
- Cohen, T.; Jenkins, H.E.; Lu, C.; McLaughlin, M.; Floyd, K.; Zignol, M. On the spread and control of MDR-TB epidemics: An examination of trends in anti-tuberculosis drug resistance surveillance data. Drug Resist. Updat. 2014, 17, 105–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.; Shukla, P.; Huang, W.; Hu, N. Gene mutations in Mycobacterium tuberculosis: Multidrug-resistant TB as an emerging global public health crisis. Tuberculosis 2015, 95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Noor, R.; Akhter, S.; Rahman, F.; Munshi, S.K.; Kamal, S.M.M.; Feroz, F. Frequency of extensively drug-resistant tuberculosis (XDR-TB) among re-treatment cases in NIDCH, Dhaka, Bangladesh. J. Infect. Chemother. 2013, 19, 243–248. [Google Scholar] [CrossRef]
- Parida, S.K.; Axelsson-Robertson, R.; Rao, M.V.; Singh, N.; Master, I.; Lutckii, A.; Keshavjee, S.; Andersson, J.; Zumla, A.; Maeurer, M. Totally drug-resistant tuberculosis and adjunct therapies. J. Intern. Med. 2015, 277, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, S.; Munoz-Torrico, M.; Duarte, R.; Dalcolmo, M.; D’Ambrosio, L.; Migliori, G.-B. New drugs and perspectives for new anti-tuberculosis regimens. Pulmonology 2018, 24, 86–98. [Google Scholar] [CrossRef]
- Kumar, K.; Kon, O.M. Diagnosis and treatment of tuberculosis: Latest developments and future priorities. Ann. Res. Hosp. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Santos, N.C.S.; Scodro, R.B.L.; de Almeida, A.L.; Baldin, V.P.; Nakamura de Vasconcelos, S.S.; Siqueira, V.L.D.; Caleffi-Ferracioli, K.R.; Campanerut-Sa, P.A.Z.; Cardoso, R.F. Combinatory activity of linezolid and levofloxacin with antituberculosis drugs in Mycobacterium tuberculosis. Tuberculosis 2018, 111, 41–44. [Google Scholar] [CrossRef]
- Francis, J.K.; Higgins, E. Permanent peripheral neuropathy: A case report on a rare but serious debilitating side-effect of fluoroquinolone administration. J. Investig. Med. High Impact Case Rep. 2014, 2, 2324709614545225. [Google Scholar] [CrossRef] [Green Version]
- Warner, D.F.; Mizrahi, V. Shortening treatment for tuberculosis—Back to basics. N. Engl. J. Med. 2014, 371, 1642–1643. [Google Scholar] [CrossRef]
- Horsburgh, C.R.; Barry, C.E.; Lange, C. Treatment of tuberculosis. N. Engl. J. Med. 2015, 373, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- McLeod, R.; Kieffer, F.; Sautter, M.; Hosten, T.; Pelloux, H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem. Inst. Oswaldo Cruz 2009, 104, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert-Gangneux, F.; Darde, M.L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Yolken, R.H. Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta Physiol. 2015, 213, 828–845. [Google Scholar] [CrossRef] [Green Version]
- Opsteegh, M.; Kortbeek, T.M.; Havelaar, A.H.; van der Giessen, J.W. Intervention strategies to reduce human Toxoplasma gondii disease burden. Clin. Infect. Dis. 2015, 60, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Eza, D.E.; Lucas, S.B. Fulminant toxoplasmosis causing fatal pneumonitis and myocarditis. HIV Med. 2006, 7, 415–420. [Google Scholar] [CrossRef]
- Paquet, C.; Yudin, M.H. Society of Obstetricians and Gynaecologists of Canada. Toxoplasmosis in pregnancy: Prevention, screening, and treatment. J. Obstet. Gynaecol. Can. 2013, 35, 78–81. [Google Scholar]
- Li, X.L.; Wei, H.X.; Zhang, H.; Peng, H.J.; Lindsay, D.S. A meta-analysis on risks of adverse pregnancy outcomes in Toxoplasma gondii infection. PLoS ONE 2014, 9, e97775. [Google Scholar] [CrossRef]
- Ballard, A.R. Toxoplasmosis. In Neonatal Infections; Cantey, J., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Neville, A.J.; Zach, S.J.; Wang, X.; Larson, J.J.; Judge, A.K.; Davis, L.A.; Vennerstrom, J.L.; Davis, P.H. Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrob. Agents Chemother. 2015, 59, 7161–7169. [Google Scholar] [CrossRef] [Green Version]
- Hopper, A.T.; Brockman, A.; Wise, A.; Gould, J.; Barks, J.; Radke, J.B.; Sibley, L.D.; Zou, Y.; Thomas, S. Discovery of selective Toxoplasma gondii dihydrofolate reductase inhibitors for the treatment of toxoplasmosis. J. Med. Chem. 2019, 62, 1562–1576. [Google Scholar] [CrossRef]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Anderson, A.C. Targeting DHFR in parasitic protozoa. Drug Discov. Today 2005, 10, 121–128. [Google Scholar]
- Porter, S.B.; Sande, M.A. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N. Engl. J. Med. 1992, 327, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wu, T.; Zhai, S.Q.; Li, C.H. Recent progress on anti-Toxoplasma drugs discovery: Design, synthesis and screening. Eur. J. Med. Chem. 2019, 183, 111711. [Google Scholar] [CrossRef]
- Abdullahi, S.A.; Unyah, N.Z.; Nordin, N.; Basir, R.; Nasir, W.M.; Alapid, A.A.; Hassan, Y.; Mustapha, T.; Majid, R.A. Therapeutic targets on Toxoplasma gondii parasite in combating toxoplasmosis. Annu. Res. Rev. Biol. 2019, 32, 49444. [Google Scholar]
- Lai, B.S.; Witola, W.H.; El Bissati, K.; Zhou, Y.; Mui, E.; Fomovska, A.; McLeod, R. Molecular target validation, antimicrobial delivery, and potential treatment of Toxoplasma gondii infections. PNAS 2012, 109, 14182–14187. [Google Scholar] [CrossRef] [Green Version]
- Angel, S.O.; Vanagas, L.; Ruiz, D.M.; Cristaldi, C.; Saldarriaga Cartagena, A.M.; Sullivan, W.J., Jr. Emerging therapeutic targets against Toxoplasma gondii: Update on DNA repair persponse inhibitors and genotoxic drugs. Front. Cell. Infect. Microbiol. 2020, 10, 289. [Google Scholar] [CrossRef]
- Agarwal, M.; Patnaik, G.; Khetan, V.; De-la-Torre, A. Ocular co-infection with Mycobacterium tuberculosis and Toxoplasma gondii in an immunocompetent patient—A case report. Ocul. Immunol. Inflamm. 2021, 30, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Dimal, N.P.M.; Santos, N.J.C.; Reyes, N.G.D.; Astejada, M.N.; Jamora, R.D.G. Hemichorea—Hemiballismus as a presentation of cerebritis from intracranial toxoplasmosis and tuberculosis. Tremor Other Hyperkinetic Mov. 2021, 11, 2. [Google Scholar] [CrossRef]
- Hwang, E.H.; Ahn, P.G.; Lee, D.M.; Kim, H.S. Cerebral toxoplasmosis combined with disseminated tuberculosis. J. Korean Neurosurg. Soc. 2012, 51, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, V.; Vaswami, R.K.; Lahiri, K.R.; Kondelar, S.S. Neurotoxoplasmosis mimicking intracranial tuberculoma. J. Postgrad. Med. 2010, 56, 31–34. [Google Scholar] [PubMed]
- Madi, D.; Achappa, B.; Rao, S.; Adhikari, P.; Makalingam, S. Cerebral toxoplasmosis mimicking intracranial tuberculoma. J. Clin. Diagn. Res. 2012, 6, 1083–1085. [Google Scholar]
- Vasantham, V.; Jahan, A.; Dogra, R.K.; Singh, S.; Singh, G.; Gupta, R.; Sarin, N. Codetection of pulmonary tuberculosis and toxoplasmosis in a pediatric bronchoalveolar lavage specimen: A cytologist’s assistance to clinical management. Diagn. Cytopathol. 2021, 49, E20–E23. [Google Scholar] [CrossRef]
- Kita, W.M.; Tume, C.B. Toxoplasma gondii and tuberculosis co-infection among tuberculosis patients at the Bamenda Regional Hospital, North West Region, Cameroon. Asian J. Res. Biochem. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Mashaly, M.; Nabih, N.; Fawzy, I.M.; El Henawy, A.A. Tuberculosis/toxoplasmosis co-infection in Egyptian patients: A reciprocal impact. Asian Pac. J. Trop. Med. 2017, 10, 315–319. [Google Scholar] [CrossRef]
- Ali, E.N.; Majeed, S.Z.; Kadhem, A.A.; Alubadi, A.E.M. Prevalence of toxoplasmosis as co-infection in Iraqi patients infected with tuberculosis. Biomed. Res. 2019, 30, 401–405. [Google Scholar]
- Zhao, Y.J.; Zhao, Y.H.; Zhang, X.Y.; Sun, X.J.; Liu, Y.Q.; Hou, Y.J.; Wu, J.Q.; Jia, H.; Han, Y.H.; Dong, W.; et al. First report of Toxoplasma gondii infection in tuberculosis patients in China. Vector Borne Zoonotic Dis. 2017, 17, 799–803. [Google Scholar] [CrossRef]
- Guneratne, R.; Mendis, D.; Bandara, T.; Fernando, S.D. Toxoplasma, Toxocara and tuberculosis co-infection in a four year old child. BMC Pediatr. 2011, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Parsaei, M.; Spotin, A.; Matini, M.; Mahjub, H.; Aghazadeh, M.; Ghahremani, G.; Taherkhani, H. Prevalence of toxoplasmosis in patients infected with tuberculosis; a sero-molecular case-control study in northwest Iran. Comp. Immunol. Microbiol. Infect. Dis. 2022, 81, 101720. [Google Scholar] [CrossRef]
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Synthesis and antimycobacterial activity of thiazolidine-2,4-dione based derivatives with halogenbenzohydrazones and pyridinecarbohydrazones substituents. Eur. J. Med. Chem. 2020, 189, 112045. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Design, synthesis and antimycobacterial activity of thiazolidine-2,4-dionebased thiosemicarbazone derivatives. Bioorg. Chem. 2020, 97, 103676. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Przekora, A.; Zalewska, J.; Ginalska, G.; Paneth, A.; Wujec, M. Synthesis and in vitro antiproliferative and antibacterial activity of new thiazolidine-2,4-dione derivatives. J. Enzyme Inhib. Med. Chem. 2018, 33, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, J.M.; Coghill, A.E.; Kim, Y.; Bender, N.; Smith-Warner, S.A.; Gapstur, S.; Teras, L.R.; Grimsrud, T.K.; Waterboer, T.; Egan, K.M. Toxoplasma gondii infection and the risk of adult glioma in two prospective studies. Int. J. Cancer 2021, 148, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.E.; Dubey, J.P. 3-Update on Toxoplasma gondii as a parasite in food: Analysis and control. Adv. Microb. Food Saf. 2015, 2, 59–80. [Google Scholar]
- Liesen, A.P.; de Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; de Araújo, J.M.; de Lima, J.G.; de Faria, A.R.; de Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar] [CrossRef]
- Mzabi, A.; Aubert, D.; Villena, I. Mechanisms of drug resistance in Toxoplasma gondii. In Antimicrobial Drug Resistance; Mayers, D.L., Sobel, J.D., Ouellette, M., Kaye, K.S., Marchaim, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1, pp. 677–684. [Google Scholar]
- Trotsko, N.; Bekier, A.; Paneth, A.; Wujec, M.; Dzitko, K. Synthesis and in vitro anti-Toxoplasma gondii activity of novel thiazolidin-4-one derivatives. Molecules 2019, 24, 3029. [Google Scholar] [CrossRef] [Green Version]
- Carradori, S.; Secci, D.; Bizzarri, B.; Chimenti, P.; De Monte, C.; Guglielmi, P.; Campestre, C.; Rivanera, D.; Bordon, C.; Jones-Brando, L. Synthesis and biological evaluation of anti-Toxoplasma gondii activity of a novel scaffold of thiazolidinone derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 746–758. [Google Scholar] [CrossRef] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Dzitko, K. Systematic identification of thiosemicarbazides for inhibition of Toxoplasma gondii growth in vitro. Molecules 2019, 24, 614. [Google Scholar] [CrossRef] [Green Version]
- Paneth, A.; Węglińska, L.; Bekier, A.; Stefaniszyn, E.; Wujec, M.; Trotsko, N.; Hawrył, A.; Hawrył, M.; Dzitko, K. Discovery of potent and selective halogen-substituted imidazole-thiosemicarbazides for inhibition of Toxoplasma gondii growth in vitro via structure-based design. Molecules 2019, 24, 1618. [Google Scholar] [CrossRef] [Green Version]
- Sanford, A.G.; Schulze, T.T.; Potluri, L.P.; Watson, G.F.; Darner, E.B.; Zach, S.J.; Hemsley, R.M.; Wallick, A.I.; Warner, R.C.; Charman, S.A.; et al. Derivatives of benzoquinone acyl hydrazone with activity against Toxoplasma gondii. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 488–492. [Google Scholar] [CrossRef] [PubMed]





| TZD Hybrid Molecules | CC30 b in Hs27 Cells (µg/mL) | IC50Tg c in Tg-Infected Hs27 Cells (µg/mL) | SI d | |
|---|---|---|---|---|
| 1 |  | 128.27 | 12.93 | 9.92 |
| 2 |  | >995.98 | 8.37 | >118.93 |
| 3 |  | >1071.05 | 22.15 | >48.35 |
| 4 |  | 654.42 | 39.02 | 16.77 |
| 5 |  | >1082.10 | 24.33 | >44.48 |
| 6 |  | >1193.22 | 27.24 | >43.81 |
| 7 |  | >880.97 | 5.94 | >148.28 |
| 8 |  | >967.09 | 8.74 | >110.67 |
| 9 |  | >1078.21 | 12.29 | >87.75 |
| 10 |  | 312.27 | 5.32 | 58.71 |
| 11 |  | >951.00 | 12.07 | >78.81 |
| 12 |  | >962.05 | 10.17 | >94.59 |
| ATO |  | 54.26 | 0.24 | 227.55 |
| PYR |  | 49.62 | 2.86 | 17.38 |
| TRI |  | 81.82 | 14.79 | 5.53 |
| SDZ |  | 2230.61 | 1254.36 | 1.78 |
| TZD Hybrid Molecules | Hemolysis (%) |
|---|---|
| 2 | 0.68 ± 0.09 |
| 7 | 0.73 ± 0.1 |
| 8 | 0.69 ± 0.04 |
| 10 | 0.66 ± 0.02 |
| Untreated cells | 0.59 ± 0.06 |
| Triton-X (0.1%) | 99.85 ± 1.65 **** |
| TZD Hybrid Molecules | PAMPA-BBB Pe (×10−6 cm·s−1) a |
|---|---|
| 2 | 12.45 ± 1.02 |
| 7 | 23.05 ± 0.71 |
| 8 | 26.11 ± 1.30 |
| 10 | 24.37 ± 0.93 |
| IC50 (µg/mL) ± SD | ||||
|---|---|---|---|---|
| 2 | 7 | 8 | 10 | Temozolomide |
| 19.36 ± 1.13 | >100 | >100 | 94.57 ± 6.16 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzitko, K.; Kaproń, B.; Paneth, A.; Bekier, A.; Plech, T.; Paneth, P.; Trotsko, N. TZD-Based Hybrid Molecules Act as Dual Anti-Mycobacterium tuberculosis and Anti-Toxoplasma gondii Agents. Int. J. Mol. Sci. 2023, 24, 2069. https://doi.org/10.3390/ijms24032069
Dzitko K, Kaproń B, Paneth A, Bekier A, Plech T, Paneth P, Trotsko N. TZD-Based Hybrid Molecules Act as Dual Anti-Mycobacterium tuberculosis and Anti-Toxoplasma gondii Agents. International Journal of Molecular Sciences. 2023; 24(3):2069. https://doi.org/10.3390/ijms24032069
Chicago/Turabian StyleDzitko, Katarzyna, Barbara Kaproń, Agata Paneth, Adrian Bekier, Tomasz Plech, Piotr Paneth, and Nazar Trotsko. 2023. "TZD-Based Hybrid Molecules Act as Dual Anti-Mycobacterium tuberculosis and Anti-Toxoplasma gondii Agents" International Journal of Molecular Sciences 24, no. 3: 2069. https://doi.org/10.3390/ijms24032069