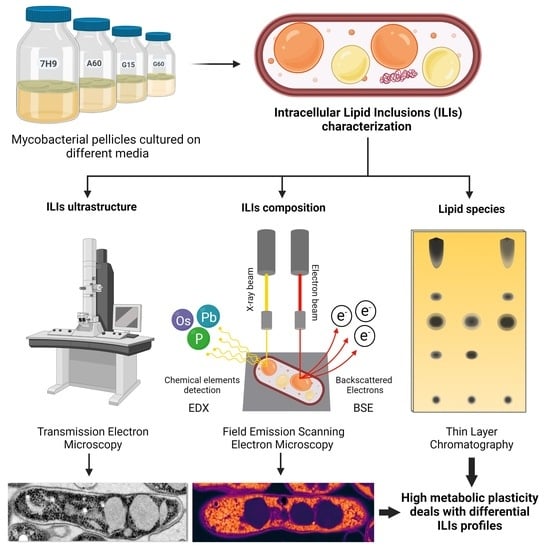
The High Plasticity of Nonpathogenic Mycobacterium brumae Induces Rapid Changes in Its Lipid Profile during Pellicle Maturation: The Potential of This Bacterium as a Versatile Cell Factory for Lipid Compounds of Therapeutic Interest
Abstract
:1. Introduction
2. Results
2.1. Pellicle Maturation Induces Large Modifications to the Lipid Composition of M. brumae
2.2. Pellicle Maturation Induces Changes in the Amount of ILIs and Their Ultrastructure in M. brumae Cells
2.3. Ultrastructural Characterization of the ILIs from Mature Pellicles
2.4. Compositional Characterization of the ILIs and Cytoplasm from Mature Pellicles
2.4.1. Unsaturated Lipid Distribution
2.4.2. Distribution and Quantification of Unsaturated Lipids and Polar Compounds
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Lipid Profiles of the Pellicles
4.3. Ultrastructural Morphometry of the Mycobacterial Pellicles
4.4. Compositional Analyses of the Mycobacterial Cells
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, M. The Mycobacterial Cell Envelope—Lipids. Cold Spring Harb. Perspect. Med. 2014, 4, a021105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallick, I.; Santucci, P.; Poncin, I.; Point, V.; Kremer, L.; Cavalier, J.F.; Canaan, S. Intrabacterial Lipid Inclusions in Mycobacteria: Unexpected Key Players in Survival and Pathogenesis? FEMS Microbiol. Rev. 2021, 45, fuab029. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Z.; Canaan, S. Biology of Mycobacterial Lipids; Academic Press: Cambridge, MA, USA, 2022; ISBN 9780323919487. [Google Scholar]
- Dargham, T.; Mallick, I.; Raze, D.; Kremer, L.; Canaan, S. Intrabacterial Lipid Inclusions: Overview of an Amazing Organelle. Biol. Mycobact. Lipids 2022, 253–269. [Google Scholar] [CrossRef]
- Burdon, K.L. Disparity in Appearance of True Hansen’s Bacilli and Cultured Leprosy Bacilli When Stained for Fat. J. Bacteriol. 1946, 52, 679. [Google Scholar] [CrossRef] [Green Version]
- Brieger, E.M.; Glauert, A.M. Electron Microscopy of the Leprosy Bacillus: A Study of Submicroscopical Structure. Tubercle 1956, 37, 195–206. [Google Scholar] [CrossRef]
- Viljoen, A.; Blaise, M.; de Chastellier, C.; Kremer, L. MAB_3551c Encodes the Primary Triacylglycerol Synthase Involved in Lipid Accumulation in Mycobacterium abscessus. Mol. Microbiol. 2016, 102, 611–627. [Google Scholar] [CrossRef]
- Schaefer, W.B.; Lewis, C.W., Jr. Effect of Oleic Acid on Growth and Cell Structure of Mycobacteria. J. Bacteriol. 1965, 90, 1438–1447. [Google Scholar] [CrossRef] [Green Version]
- Garton, N.J.; Christensen, H.; Minnikin, D.E.; Adegbola, R.A.; Barer, M.R. Intracellular Lipophilic Inclusions of Mycobacteria in Vitro and in Sputum. Microbiology 2002, 148, 2951–2958. [Google Scholar] [CrossRef] [Green Version]
- Nohynek, L.J.; Häggblom, M.M.; Palleroni, N.J.; Kronqvist, K.; Nurmiaho-Lassila, E.L.; Salkinoja-Salonen, M. Characterization of a Mycobacterium fortuitum Strain Capable of Degrading Polychlorinated Phenolic Compounds. Syst. Appl. Microbiol. 1993, 16, 126–134. [Google Scholar] [CrossRef]
- Luquin, M.; Ausina, V.; Vincent-Levy-Frebault, V.; Laneelle, M.A.; Belda, F.; Garcia-Barcelo, M.; Prats, G.; Daffe, M. Mycobacterium brumae Sp. Nov., a Rapidly Growing, Nonphotochromogenic Mycobacterium. Int. J. Syst. Bacteriol. 1993, 43, 405–413. [Google Scholar] [CrossRef]
- Noguera-Ortega, E.; Secanella-Fandos, S.; Eraña, H.; Gasión, J.; Rabanal, R.M.; Luquin, M.; Torrents, E.; Julián, E. Nonpathogenic Mycobacterium brumae Inhibits Bladder Cancer Growth In Vitro, Ex Vivo, and In Vivo. Eur. Urol. Focus 2016, 2, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bach-Griera, M.; Campo-Pérez, V.; Barbosa, S.; Traserra, S.; Guallar-Garrido, S.; Moya-Andérico, L.; Herrero-Abadía, P.; Luquin, M.; Rabanal, R.M.; Torrents, E.; et al. Mycolicibacterium brumae Is a Safe and Non-Toxic Immunomodulatory Agent for Cancer Treatment. Vaccines 2020, 8, 198. [Google Scholar] [CrossRef]
- Guallar-Garrido, S.; Ez, V.; Sánchez-Chardi, A.; Luquin, M.; Julián, E. Each Mycobacterium Requires a Specific Culture Medium Composition for Triggering an Optimized Immunomodulatory and Antitumoral Effect. Microorganisms 2020, 8, 734. [Google Scholar] [CrossRef]
- Sturley, S.L.; Hussain, M.M. Lipid Droplet Formation on Opposing Sides of the Endoplasmic Reticulum. J. Lipid Res. 2012, 53, 1800–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirakova, T.D.; Deb, C.; Daniel, J.; Singh, H.D.; Maamar, H.; Dubey, V.S.; Kolattukudy, P.E. Wax Ester Synthesis Is Required for Mycobacterium tuberculosis to Enter in Vitro Dormancy. PLoS ONE 2012, 7, e51641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, S.K.; Heintz, J.A.; Albrecht, R.M.; Talaat, A.M. Single-Cell Elemental Analysis of Bacteria: Quantitative Analysis of Polyphosphates in Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2012, 2, 63. [Google Scholar] [CrossRef] [Green Version]
- Mali, P.C.; Meena, L.S. Triacylglycerol: Nourishing Molecule in Endurance of Mycobacterium tuberculosis. J. Biosci. 2018, 43, 149–154. [Google Scholar] [CrossRef]
- Cook, G.M.; Berney, M.; Gebhard, S.; Heinemann, M.; Cox, R.A.; Danilchanka, O.; Niederweis, M. Physiology of Mycobacteria. Adv. Microb. Physiol. 2009, 55, 81–319. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J.D.; Bosmani, C.; Barisch, C.; Raykov, L.; Lefrançois, L.H.; Cardenal-Muñoz, E.; López-Jiménez, A.T.; Soldati, T. Eat Prey, Live: Dictyostelium discoideum as a Model for Cell-Autonomous Defenses. Front. Immunol. 2018, 8, 1906. [Google Scholar] [CrossRef] [Green Version]
- Barisch, C.; López-Jiménez, A.T.; Soldati, T. Live Imaging of Mycobacterium marinum Infection in Dictyostelium discoideum. Methods Mol. Biol. 2015, 1285, 369–385. [Google Scholar] [CrossRef]
- Wältermann, M.; Hinz, A.; Robenek, H.; Troyer, D.; Reichelt, R.; Malkus, U. Mechanism of Lipid-Body Formation in Prokaryotes: How Bacteria Fatten Up. Mol. Microbiol. 2005, 55, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J. The Biogenesis and Functions of Lipid Bodies in Animals, Plants and Microorganisms. Prog. Lipid Res. 2001, 40, 325–438. [Google Scholar] [CrossRef]
- Jain, M.; Petzold, C.J.; Schelle, M.W.; Leavell, M.D.; Mougous, J.D.; Bertozzi, C.R.; Leary, J.A.; Cox, J.S. Lipidomics Reveals Control of Mycobacterium tuberculosis Virulence Lipids via Metabolic Coupling. Proc. Natl. Acad. Sci. USA 2007, 104, 5133–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walpole, G.F.W.; Grinstein, S.; Westman, J. The Role of Lipids in Host-Pathogen Interactions. IUBMB Life 2018, 70, 384–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stehr, M.; Elamin, A.A.; Singh, M. Lipid Inclusions in Mycobacterial Infections; Books on Demand: Norderstedt, Germany, 2013; ISBN 978-953-51-1049-1. [Google Scholar]
- Barisch, C.; Paschke, P.; Hagedorn, M.; Maniak, M.; Soldati, T. Lipid Droplet Dynamics at Early Stages of Mycobacterium marinum Infection in Dictyostelium. Cell. Microbiol. 2015, 17, 1332–1349. [Google Scholar] [CrossRef]
- Barisch, C.; Soldati, T. Mycobacterium Marinum Degrades Both Triacylglycerols and Phospholipids from Its Dictyostelium Host to Synthesise Its Own Triacylglycerols and Generate Lipid Inclusions. PLoS Pathog. 2017, 13, e1006095. [Google Scholar] [CrossRef] [Green Version]
- Neyrolles, O.; Hernández-Pando, R.; Pietri-Rouxel, F.; Fornès, P.; Tailleux, L.; Payán, J.A.B.; Pivert, E.; Bordat, Y.; Aguilar, D.; Prévost, M.-C.; et al. Is Adipose Tissue a Place for Mycobacterium tuberculosis Persistence? PLoS ONE 2006, 1, e43. [Google Scholar] [CrossRef] [Green Version]
- Falkinham, J.O. The Biology of Environmental Mycobacteria. Environ. Microbiol. Rep. 2009, 1, 477–487. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, A.; Biswas, P.; Bhatt, A.; Visweswariah, S.S. Mycobacterial Phenolic Glycolipid Synthesis Is Regulated by CAMP-Dependent Lysine Acylation of FadD22. Microbiology 2017, 163, 373–382. [Google Scholar] [CrossRef]
- Bailo, R.; Bhatt, A.; Aínsa, J.A. Lipid Transport in Mycobacterium tuberculosis and Its Implications in Virulence and Drug Development. Biochem. Pharmacol. 2015, 96, 159–167. [Google Scholar] [CrossRef]
- Graham, L.; Orenstein, J.M. Processing Tissue and Cells for Transmission Electron Microscopy in Diagnostic Pathology and Research. Nat. Protoc. 2007, 2, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.E.; Stirling, J.W. Electron Microscopy. Theory Pract. Histol. Tech. 2008, 601–640. [Google Scholar] [CrossRef]
- Maurya, R.K.; Bharti, S.; Krishnan, M.Y. Triacylglycerols: Fuelling the Hibernating Mycobacterium tuberculosis. Front. Cell. Infect. Microbiol. 2019, 8, 450. [Google Scholar] [CrossRef]
- Vijay, S.; Hai, H.T.; Thu, D.D.A.; Johnson, E.; Pielach, A.; Phu, N.H.; Thwaites, G.E.; Thuong, N.T.T. Ultrastructural Analysis of Cell Envelope and Accumulation of Lipid Inclusions in Clinical Mycobacterium tuberculosis Isolates from Sputum, Oxidative Stress, and Iron Deficiency. Front. Microbiol. 2018, 8, 2681. [Google Scholar] [CrossRef] [PubMed]
- Taher, S.G.; Al Dulayymi, J.R.; Tima, H.G.; Ali, H.M.; Romano, M.; Baird, M.S. Synthesis of Wax Esters and Related Trehalose Esters from Mycobacterium avium and Other Mycobacteria. Tetrahedron 2016, 72, 3863–3876. [Google Scholar] [CrossRef]
- Ishige, T.; Tani, A.; Sakai, Y.; Kato, N. Wax Ester Production by Bacteria. Curr. Opin. Microbiol. 2003, 6, 244–250. [Google Scholar] [CrossRef]
- Rontani, J.-F. Production of Wax Esters by Bacteria. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 459–470. [Google Scholar]
- Gouzy, A.; Larrouy-Maumus, G.; Bottai, D.; Levillain, F.; Dumas, A.; Wallach, J.B.; Caire-Brandli, I.; de Chastellier, C.; Wu, T.D.; Poincloux, R.; et al. Mycobacterium tuberculosis Exploits Asparagine to Assimilate Nitrogen and Resist Acid Stress during Infection. PLoS Pathog. 2014, 10, e1003928. [Google Scholar] [CrossRef] [Green Version]
- Fixter, L.M.; Nagi, M.N.; McCormack, J.G.; Fewson, C.A. Structure, Distribution and Function of Wax Esters in Acinetobacter calcoaceticus. J. Gen. Microbiol. 1986, 132, 3147–3157. [Google Scholar] [CrossRef] [Green Version]
- Waltermann, M.; Steinbuchel, A. Neutral Lipid Bodies in Prokaryotes: Recent Insights into Structure, Formation, and Relationship to Eukaryotic Lipid Depots. J. Bacteriol. 2005, 187, 3607–3619. [Google Scholar]
- Steinbüchel, A.; Wältermann, M. Wax Ester and Triacylglycerol Inclusions. In Bacterial Organelles and Organelle-Like Inclusions; Springer: Cham, Switzerland, 2020; pp. 211–242. [Google Scholar] [CrossRef]
- Sartain, M.J.; Dick, D.L.; Rithner, C.D.; Crick, D.C.; Belisle, J.T. Lipidomic Analyses of Mycobacterium tuberculosis Based on Accurate Mass Measurements and the Novel “Mtb LipidDB”. J. Lipid Res. 2011, 52, 861–872. [Google Scholar] [CrossRef]
- Alvarez, H.M.; Steinbüchel, A. Triacylglycerols in Prokaryotic Microorganisms. Appl. Microbiol. Biotechnol. 2002, 60, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Pacífico, C.; Fernandes, P.; de Carvalho, C.C.C.R. Mycobacterial Response to Organic Solvents and Possible Implications on Cross-Resistance with Antimicrobial Agents. Front. Microbiol. 2018, 9, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, J.; Deb, C.; Dubey, V.S.; Sirakova, T.D.; Abomoelak, B.; Morbidoni, H.R.; Kolattukudy, P.E. Induction of a Novel Class of Diacylglycerol Acyltransferases and Triacylglycerol Accumulation in Mycobacterium tuberculosis as It Goes into a Dormancy-like State in Culture. J. Bacteriol. 2004, 186, 5017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deb, C.; Daniel, J.; Sirakova, T.D.; Abomoelak, B.; Dubey, V.S.; Kolattukudy, P.E. A Novel Lipase Belonging to the Hormone-Sensitive Lipase Family Induced under Starvation to Utilize Stored Triacylglycerol in Mycobacterium tuberculosis. J. Biol. Chem. 2006, 281, 3866–3875. [Google Scholar] [CrossRef] [Green Version]
- Santucci, P.; Johansen, M.D.; Point, V.; Poncin, I.; Viljoen, A.; Cavalier, J.F.; Kremer, L.; Canaan, S. Nitrogen Deprivation Induces Triacylglycerol Accumulation, Drug Tolerance and Hypervirulence in Mycobacteria. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dow, A.; Prisic, S. Alternative Ribosomal Proteins Are Required for Growth and Morphogenesis of Mycobacterium smegmatis under Zinc Limiting Conditions. PLoS ONE 2018, 13, e0196300. [Google Scholar] [CrossRef] [Green Version]
- Orlovich, D.A.; Ashford, A.E. Polyphosphate Granules Are an Artefact of Specimen Preparation in the Ectomycorrhizal Fungus Pisolithus tinctorius. Protoplasma 1993, 173, 91–102. [Google Scholar] [CrossRef]
- Bleck, C.K.; Merz, A.; Gutierrez, M.G.; Walther, P.; Dubochet, J.; Zuber, B. Comparison of Different Methods for Thin Section EM Analysis of Mycobacterium smegmatis. J. Microsc. 2010, 237, 23–38. [Google Scholar] [CrossRef]
- Velayati, A.A.; Farnia, P.; Ibrahim, T.A.; Haroun, R.Z.; Kuan, H.O.; Ghanavi, J.; Farnia, P.; Kabarei, A.N.; Tabarsi, P.; Omar, A.R.; et al. Differences in Cell Wall Thickness between Resistant and Nonresistant Strains of Mycobacterium tuberculosis: Using Transmission Electron Microscopy. Chemotherapy 2009, 55, 303–307. [Google Scholar] [CrossRef]
- Yamada, H.; Yamaguchi, M.; Igarashi, Y.; Chikamatsu, K.; Aono, A.; Murase, Y.; Morishige, Y.; Takaki, A.; Chibana, H.; Mitarai, S. Mycolicibacterium smegmatis, Basonym Mycobacterium smegmatis, Expresses Morphological Phenotypes Much More Similar to Escherichia coli than Mycobacterium tuberculosis in Quantitative Structome Analysis and CryoTEM Examination. Front. Microbiol. 2018, 9, 1992. [Google Scholar] [CrossRef]
- Secanella-Fandos, S.; Noguera-Ortega, E.; Olivares, F.; Luquin, M.; Julián, E. Killed but Metabolically Active Mycobacterium bovis Bacillus Calmette-Guérin Retains the Antitumor Ability of Live Bacillus Calmette-Guérin. J. Urol. 2014, 191, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campo-Pérez, V.; Guallar-Garrido, S.; Luquin, M.; Sánchez-Chardi, A.; Julián, E. The High Plasticity of Nonpathogenic Mycobacterium brumae Induces Rapid Changes in Its Lipid Profile during Pellicle Maturation: The Potential of This Bacterium as a Versatile Cell Factory for Lipid Compounds of Therapeutic Interest. Int. J. Mol. Sci. 2022, 23, 13609. https://doi.org/10.3390/ijms232113609
Campo-Pérez V, Guallar-Garrido S, Luquin M, Sánchez-Chardi A, Julián E. The High Plasticity of Nonpathogenic Mycobacterium brumae Induces Rapid Changes in Its Lipid Profile during Pellicle Maturation: The Potential of This Bacterium as a Versatile Cell Factory for Lipid Compounds of Therapeutic Interest. International Journal of Molecular Sciences. 2022; 23(21):13609. https://doi.org/10.3390/ijms232113609
Chicago/Turabian StyleCampo-Pérez, Víctor, Sandra Guallar-Garrido, Marina Luquin, Alejandro Sánchez-Chardi, and Esther Julián. 2022. "The High Plasticity of Nonpathogenic Mycobacterium brumae Induces Rapid Changes in Its Lipid Profile during Pellicle Maturation: The Potential of This Bacterium as a Versatile Cell Factory for Lipid Compounds of Therapeutic Interest" International Journal of Molecular Sciences 23, no. 21: 13609. https://doi.org/10.3390/ijms232113609