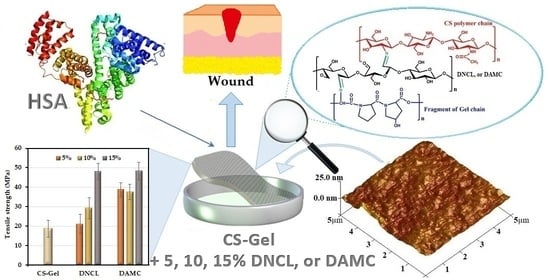
Chitosan-Gelatin Films Cross-Linked with Dialdehyde Cellulose Nanocrystals as Potential Materials for Wound Dressings
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Cellulose Nanocrystals
2.2. Properties of Cellulose Nanocrystals
2.3. Preparation of Dialdehyde Cellulose Nanocrystals
2.4. Properties of Dialdehyde Cellulose Nanocrystals
2.5. Preparation of Cross-Linked Chitosan-Gelatin Films
2.5.1. Degree of Cross-Linking
2.5.2. ATR-FTIR Spectroscopy
2.5.3. Apparent Density
2.5.4. AFM
2.5.5. Antioxidant Activity
2.5.6. Oxygen Permeability
2.5.7. The Water Vapor Transmission Rate (WVTR)
2.5.8. Toxicity Studies
2.5.9. Human Serum Albumin Adsorption Study
2.5.10. Anti-Inflammatory Study
2.5.11. Tensile Properties
2.5.12. Swelling and Degradation Rate
2.5.13. Surface Free Energy and Wettability Characteristics
3. Materials and Methods
3.1. Materials
3.2. Preparation of Cellulose Nanocrystals
3.3. Preparation of Dialdehyde Cellulose Nanocrystals
3.4. Preparation of Cross-Linked Chitosan-Gelatin Films
3.5. Properties of Cellulose Nanocrystals, Dialdehyde Cellulose Nanocrystals, and Cross-Linked Biofilms
3.5.1. Content of Aldehyde Groups
3.5.2. Particle Size Distribution
3.5.3. Thermogravimetry
3.5.4. Cross-Linking Degree and Apparent Density
3.5.5. ATR-FTIR Spectroscopy and X-ray Diffraction
3.5.6. Morphology Analysis
3.5.7. Antioxidant Activity
3.5.8. Oxygen Permeability
3.5.9. The Water Vapor Transmission Rate (WVTR)
3.5.10. Toxicity Studies
3.5.11. Human Serum Albumin Adsorption Study
3.5.12. Anti-Inflammatory Studies
3.5.13. Tensile Properties
3.5.14. Swelling and Degradation Rate
3.5.15. Surface Free Energy and Wettability Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blaker, J.J.; Lee, K.Y.; Mantalaris, A.; Bismarck, A. Ice-microsphere templating to produce highly porous nanocomposite PLA matrix scaffolds with pores selectively lined by bacterial cellulose nano-whiskers. Compos. Sci. Technol. 2010, 70, 1879–1888. [Google Scholar] [CrossRef]
- Sirin, H.; Kodal, M.; Karaagac, B.; Ozkoc, G. Effects of octamaleamic acid-POSS used as the adhesion enhancer on the properties of silicone rubber/silica nanocomposites. Compos. B. Eng. 2016, 98, 370–381. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Velasco, J.I. Viscoelastic properties of polycarbonate-graphene nanoplatelets nanocomposite foams. Compos. B. Eng. 2016, 93, 143–152. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Mlynarczyk, D.T.; Wegrzynowska-Drzymalska, K.; Ilnicka, A.; Goslinski, T.; Marszałł, M.P.; Ziegler-Borowska, M. Photosensitizing potential of tailored magnetite hybrid nanoparticles functionalized with levan and zinc (II) phthalocyanine. Appl. Surf. Sci. 2020, 524, 146602. [Google Scholar] [CrossRef]
- Sun, B.; Hou, Q.; Liu, Z.; He, Z.; Ni, Y. Stability and efficiency improvement of ASA in internal sizing of cellulosic paper by using cationically modified cellulose nanocrystals. Cellulose 2014, 21, 2879–2887. [Google Scholar] [CrossRef]
- Meesorn, W.; Shirole, A.; Vanhecke, D.; de Espinosa, L.M.; Weder, C. A Simple and Versatile Strategy To Improve the Mechanical Properties of Polymer Nanocomposites with Cellulose Nanocrystals. Macromolecules 2017, 50, 2364–2374. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and Cellular Uptake of Folic Acid-Conjugated Cellulose Nanocrystals for Cancer Targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef]
- Liu , J.; Plog, A.; Groszewicz, P.; Zhao, L.; Xu, Y.; Breitzke, H.; Stark, A.; Hoffmann, R.; Gutmann, T.; Zhang, K.; et al. Design of a Heterogeneous Catalyst Based on Cellulose Nanocrystals for Cyclopropanation: Synthesis and Solid-State NMR Characterization. Chem. Eur. J. 2015, 21, 12414–12420. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, J.; Yu, J. Surface acetylation of cellulose nanocrystal and its reinforcing function in poly(lactic acid). Carohydr. Polym. 2011, 83, 1834–1842. [Google Scholar] [CrossRef]
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A. Acid-Free Preparation of Cellulose Nanocrystals by TEMPO Oxidation and Subsequent Cavitation. Biomacromolecules 2018, 19, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.; Bras, J.; Dufresne, A. New Process of Chemical Grafting of Cellulose Nanoparticles with a Long Chain Isocyanate. Langmuir 2010, 26, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Filpponen, I.; Argyropoulos, D.S. Regular Linking of Cellulose Nanocrystals via Click Chemistry: Synthesis and Formation of Cellulose Nanoplatelet Gels. Biomacromolecules 2010, 11, 1060–1066. [Google Scholar] [CrossRef]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter. 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Imidazolium grafted cellulose nanocrystals for ion exchange applications. Chem. Commun. 2011, 47, 4177–4179. [Google Scholar] [CrossRef]
- Lu, F.-F.; Yu, H.-Y.; Zhou, Y.; Yao, J.-M. Spherical and rod-like dialdehyde cellulose nanocrystals by sodium periodate oxidation: Optimization with double response surface model and templates for silver nanoparticles. Express Polym. Lett. 2016, 10, 965–976. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Yu, X.; Gu, Z.; Zhang, X. Feasibility study of a novel crosslinking reagent (alginate dialdehyde) for biological tissue fixation. Carohydr. Polym. 2012, 87, 1589–1595. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning structure and properties of gelatin edible films through pullulan dialdehyde crosslinking. LWT 2021, 138, 110607. [Google Scholar] [CrossRef]
- Wegrzynowska-Drzymalska, K.; Grebicka, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef] [Green Version]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is dialdehyde starch a valuable cross-linking agent for collagen/elastin based materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The application of chitosan/collagen/hyaluronic acid sponge cross-linked by dialdehyde starch addition as a matrix for calcium phosphate in situ precipitation. Int. J. Biol. Macromol. 2018, 107, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Persinal-Medina, M.; Llames, S.; Chacón, M.; Vázquez, N.; Pevida, M.; Alcalde, I.; Alonso-Alonso, S.; Martínez-López, L.M.; Merayo-Lloves, J.; Meana, Á. Polymerizable Skin Hydrogel for Full Thickness Wound Healing. Int. J. Mol. Sci. 2022, 23, 4837. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Luo, G.; Xia, H.; He, W.; Zhao, J.; Liu, B.; Tan, J.; Zhou, J.; Liu, D.; Wang, Y.; et al. Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 2015, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and biodegradable chitosan/agarose film revealing slightly acidic pH for potential applications in regenerative medicine as artificial skin graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Huang, Y.; Onyeri, S.; Siewe, M.; Moshfeghian, A.; Madihally, S.V. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials 2005, 26, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S.; Hadjizadeh, A.; Niknejad, H. Chitosan-gelatin porous scaffold incorporated with Chitosan nanoparticles for growth factor delivery in tissue engineering. Carohydr. Polym. 2018, 202, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Development of Polyelectrolyte Chitosan-Gelatin Hydrogels for Skin Bioprinting. Procedia CIRP 2016, 49, 105–112. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gómez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Functional Properties of Bioplastics Made from Wheat Gliadins Modified with Cinnamaldehyde. J. Agric. Food. Chem. 2011, 59, 6689–6695. [Google Scholar] [CrossRef]
- Chen, R.-N.; Ho, H.-O.; Sheu, M.-T. Characterization of collagen matrices crosslinked using microbial transglutaminase. Biomaterials 2005, 26, 4229–4235. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and Chemical Factors Influencing the Printability of Hydrogel-based Extrusion Bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, T.; Endo, Y. Effects of Glutaraldehyde Exposure on Human Health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef]
- Rideout, K.; Teschke, K.; Dimich-Ward, H.; Kennedy, S.M. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J. Hosp. Infect. 2005, 59, 4–11. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Y. Biocompatibility of Genipin and Glutaraldehyde Cross-Linked Chitosan Materials in the Anterior Chamber of the Eye. Int. J. Mol. Sci. 2012, 13, 10970–10985. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, S.; Liu, B.; Yao, S.; Dai, Y.; Zhou, L.; Qin, C.; Fatehi, P. Sustainable Chitosan-Dialdehyde Cellulose Nanocrystal Film. Materials 2021, 14, 5851. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterials 2019, 9, 253. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.E.; Jin, H.-J. Chemical and physical reinforcement of hydrophilic gelatin film with di-aldehyde nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef]
- Song, K.; Xu, H.; Xie, K.; Yang, Y. Keratin-Based Biocomposites Reinforced and Cross-Linked with Dual-Functional Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2017, 5, 5669–5678. [Google Scholar] [CrossRef]
- Lee, H.; You, J.; Jin, H.-J.; Kwak, H.W. Chemical and physical reinforcement behavior of dialdehyde nanocellulose in PVA composite film: A comparison of nanofiber and nanocrystal. Carohydr. Polym. 2020, 232, 115771. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, Y.; Wu, Q.; Wei, Z.; Lin, X.; Chen, Y. Preparation and Characterization of Cellulose Nanocrystal Extraction From Pennisetum hydridum Fertilized by Municipal Sewage Sludge via Sulfuric Acid Hydrolysis. Front. Energy Res. 2021, 9, 774783. [Google Scholar] [CrossRef]
- Brito, B.S.L.; Pereira, F.V.; Putaux, J.-L.; Jean, B. Preparation, morphology and structure of cellulose nanocrystals from bamboo fibers. Cellulose 2012, 19, 1527–1536. [Google Scholar] [CrossRef]
- Le Normand, M.; Moriana, R.; Ek, M. Isolation and characterization of cellulose nanocrystals from spruce bark in a biorefinery perspective. Carohydr. Polym. 2014, 111, 979–987. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Z.; Sun, B.; Fatehi, P. Preparation of dialdehyde cellulose nanocrystal as an adsorbent for creatinine. Can. J. Chem. Eng. 2016, 94, 1435–1441. [Google Scholar] [CrossRef]
- Carrillo, I.; Mendonça, R.T.; Ago, M.; Rojas, O.J. Comparative study of cellulosic components isolated from different Eucalyptus species. Cellulose 2018, 25, 1011–1029. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; Zhang, Y. Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carohydr. Polym. 2017, 157, 945–952. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Anand Kishore, K. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Xing, X.; Han, Y.; Jiang, Q.; Sun, Y.; Wang, X.; Qu, G.; Sun, G.; Li, Y. Immobilization of laccases onto cellulose nanocrystals derived from waste newspaper: Relationship between immobilized laccase activity and dialdehyde content. Cellulose 2021, 28, 4793–4805. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Xu, Y.; Wang, A.; Xu, Z.; Wu, X.; Li, D.; Mu, C.; Ge, L. Dihydromyricetin-Loaded Pickering Emulsions Stabilized by Dialdehyde Cellulose Nanocrystals for Preparation of Antioxidant Gelatin–Based Edible Films. Food Bioprocess Technol. 2021, 14, 1648–1661. [Google Scholar] [CrossRef]
- Chauhan, K.; Kaur, J.; Kumari, A.; Kumari, A.; Chauhan, G.S. Efficient method of starch functionalization to bis-quaternary structure unit. Int. J. Biol. Macromol. 2015, 80, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, T.; Yu, S.; Zhang, Y.; Lyu, B. Preparation and application of dialdehyde nanocellulose reinforced jatropha oil based polymer emulsions as leather fatliquors. Cellulose 2021, 28, 331–346. [Google Scholar] [CrossRef]
- Nam, J.; Hyun, Y.; Oh, S.; Park, J.; Jin, H.-J.; Kwak, H.W. Effect of cross-linkable bacterial cellulose nanocrystals on the physicochemical properties of silk sericin films. Polym. Test. 2021, 97, 107161. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M.; Węgrzynowska-Drzymalska, K.; Burkowska-But, A. The Use of Chitosan and Starch-Based Flocculants for Filter Backwash Water Treatment. Materials 2022, 15, 1056. [Google Scholar] [CrossRef]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Lu, B.; Wang, T.; Li, Z.; Dai, F.; Lv, L.; Tang, F.; Yu, K.; Liu, J.; Lan, G. Healing of skin wounds with a chitosan–gelatin sponge loaded with tannins and platelet-rich plasma. Int. J. Biol. Macromol. 2016, 82, 884–891. [Google Scholar] [CrossRef]
- S, P.; Jaiswal, A.K. Effect of interpolymer complex formation between chondroitin sulfate and chitosan-gelatin hydrogel on physico-chemical and rheological properties. Carohydr. Polym. 2020, 238, 116179. [Google Scholar] [CrossRef]
- Song, Y.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C 2018, 88, 1–12. [Google Scholar] [CrossRef]
- Loukelis, K.; Papadogianni, D.; Chatzinikolaidou, M. Kappa-carrageenan/chitosan/gelatin scaffolds enriched with potassium chloride for bone tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1720–1730. [Google Scholar] [CrossRef]
- Li, M.; Aveyard, J.; Doherty, K.G.; Deller, R.C.; Williams, R.L.; Kolegraff, K.N.; Kaye, S.B.; D’Sa, R.A. Antimicrobial Nitric Oxide-Releasing Electrospun Dressings for Wound Healing Applications. ACS Mater. Au 2022, 2, 190–203. [Google Scholar] [CrossRef]
- Liu, Y.; An, M.; Wang, L.; Qiu, H. Preparation and Characterization of Chitosan-Gelatin/Glutaraldehyde Scaffolds. J. Macromol. Sci. B 2014, 53, 309–325. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan and gelatin based biodegradable packaging films with UV-light protection. J. Photochem. Photobiol. B Biol. 2016, 163, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yao, Y.; Yim, E.K.F. The effects of surface topography modification on hydrogel properties. APL Bioeng. 2021, 5, 031509. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Zhang, J.; Yao, J. Molecular interactions in gelatin/chitosan composite films. Food Chem. 2017, 235, 45–50. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Xu, K.; Fan, Z.; Wang, P.; Xu, Z.; Ren, X.; Hu, S.; Gao, Z. Fabrication of double crosslinked chitosan/gelatin membranes with Na+ and pH dual-responsive controlled permeability. Carohydr. Polym. 2020, 236, 115963. [Google Scholar] [CrossRef]
- Bölgen, N.; Demir, D.; Yalçın, M.S.; Özdemir, S. Development of Hypericum perforatum oil incorporated antimicrobial and antioxidant chitosan cryogel as a wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1581–1590. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Kan, J.; Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Liu, J. Development of active packaging based on chitosan-gelatin blend films functionalized with Chinese hawthorn (Crataegus pinnatifida) fruit extract. Int. J. Biol. Macromol. 2019, 140, 384–392. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Valachová, K.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Šoltés, L. Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol. 2021, 166, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-modified poly(vinyl alcohol)-crosslinked chitosan hydrogel films for potential wound dressing. Carohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wittaya-areekul, S.; Prahsarn, C.; Sungthongjeen, S. Development and in vitro evaluation of chitosan-Eudragit RS 30D composite wound dressings. AAPS PharmSciTech 2006, 7, E215–E220. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-H. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Bajpai, M.; Bajpai, S.K.; Gautam, D. Investigation of Regenerated Cellulose/Poly(acrylic acid) Composite Films for Potential Wound Healing Applications: A Preliminary Study. J. Appl. Chem. 2014, 2014, 325627. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef]
- Niemirycz, E.; Nichthauser, J.; Staniszewska, M.; Nałęcz-Jawecki, G.; Bolałek, J. The Microtox® biological test: Application in toxicity evaluation of surface waters and sediments in Poland. Oceanol. Hydrobiol. Stud. 2007, 36, 151–163. [Google Scholar] [CrossRef]
- Maliński, M.P.; Budzianowski, J.; Kikowska, M.; Derda, M.; Jaworska, M.M.; Mlynarczyk, D.T.; Szukalska, M.; Florek, E.; Thiem, B. Two Ecdysteroids Isolated from Micropropagated Lychnis flos-cuculi and the Biological Activity of Plant Material. Molecules 2021, 26, 904. [Google Scholar] [CrossRef]
- Barra, A.; Alves, Z.; Ferreira, N.M.; Martins, M.A.; Oliveira, H.; Ferreira, L.P.; Cruz, M.M.; Carvalho, M.d.D.; Neumayer, S.M.; Rodriguez, B.J.; et al. Biocompatible chitosan-based composites with properties suitable for hyperthermia therapy. J. Mater. Chem. B 2020, 8, 1256–1265. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A. Design of Acacia Gum–Carbopol–Cross-Linked-Polyvinylimidazole Hydrogel Wound Dressings for Antibiotic/Anesthetic Drug Delivery. Ind. Eng. Chem. Res. 2016, 55, 9176–9188. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Akdoğan, E. Protein Adsorption on Poly-3-hydroxybutyrate and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Adsorption Isotherms and Conformational Analysis of the Adsorbed Protein. Hacettepe J. Biol. Chem. 2022, 50, 163–171. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Sakthiguru, N.; Sithique, M.A. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int. J. Biol. Macromol. 2020, 152, 873–883. [Google Scholar] [CrossRef]
- Ní Annaidh, A.; Bruyère, K.; Destrade, M.; Gilchrist, M.D.; Otténio, M. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Genevro, G.M.; Gomes Neto, R.J.; de Almeida Paulo, L.; Lopes, P.S.; de Moraes, M.A.; Beppu, M.M. Glucomannan asymmetric membranes for wound dressing. J. Mater. Res. 2019, 34, 481–489. [Google Scholar] [CrossRef]
- Ho, T.T.-P.; Doan, V.K.; Tran, N.M.-P.; Nguyen, L.K.-K.; Le, A.N.-M.; Ho, M.H.; Trinh, N.-T.; Van Vo, T.; Tran, L.D.; Nguyen, T.-H. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater. Sci. Eng. C 2021, 120, 111724. [Google Scholar] [CrossRef]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J. Effect of plasticizer and surfactant on the properties of poly(vinyl alcohol)/chitosan films. Int. J. Biol. Macromol. 2020, 164, 2100–2107. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Sanchez, J.-Y.; Dufresne, A. Cellulose nanocrystals reinforced poly(oxyethylene). Polymer 2004, 45, 4149–4157. [Google Scholar] [CrossRef]
- Dong, F.; Li, S. Wound Dressings Based on Chitosan-Dialdehyde Cellulose Nanocrystals-Silver Nanoparticles: Mechanical Strength, Antibacterial Activity and Cytotoxicity. Polymers 2018, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Kharazian, N.; Izadi-Vasafi, H. Preparation and characterization of chitosan/gelatin/nanocrystalline cellulose/calcium peroxide films for potential wound dressing applications. Int. J. Biol. Macromol. 2019, 133, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.Á.V.; da C Amaro Martins, V.; de Guzzi Plepis, A.M. Chitosan/gelatin films with jatobá resin: Control of properties by vegetal resin inclusion and degree of acetylation modification. Int. J. Biol. Macromol. 2021, 182, 1737–1745. [Google Scholar] [CrossRef]
- Cui, L.; Jia, J.; Guo, Y.; Liu, Y.; Zhu, P. Preparation and characterization of IPN hydrogels composed of chitosan and gelatin cross-linked by genipin. Carohydr. Polym. 2014, 99, 31–38. [Google Scholar] [CrossRef]
- Ranjbar, J.; Koosha, M.; Chi, H.; Ghasemi, A.; Zare, F.; Abdollahifar, M.A.; Darvishi, M.; Li, T. Novel chitosan/gelatin/oxidized cellulose sponges as absorbable hemostatic agents. Cellulose 2021, 28, 3663–3675. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Maiti, S.; Khillar, P.S.; Mishra, D.; Nambiraj, N.A.; Jaiswal, A.K. Physical and self–crosslinking mechanism and characterization of chitosan-gelatin-oxidized guar gum hydrogel. Polym. Test. 2021, 97, 107155. [Google Scholar] [CrossRef]
- Ghaffari-Bohlouli, P.; Zahedi, P.; Shahrousvand, M. Enhanced osteogenesis using poly (l-lactide-co-d, l-lactide)/poly (acrylic acid) nanofibrous scaffolds in presence of dexamethasone-loaded molecularly imprinted polymer nanoparticles. Int. J. Biol. Macromol. 2020, 165, 2363–2377. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials—Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
- Whu, S.W.; Hung, K.-C.; Hsieh, K.-H.; Chen, C.-H.; Tsai, C.-L.; Hsu, S.-h. In vitro and in vivo evaluation of chitosan–gelatin scaffolds for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Zhu, X.; Zhu, W.; Li, X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour. Bioprocess. 2019, 6, 45. [Google Scholar] [CrossRef]
- Wegrzynowska-Drzymalska, K.; Mylkie, K.; Nowak, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Dialdehyde Starch Nanocrystals as a Novel Cross-Linker for Biomaterials Able to Interact with Human Serum Proteins. Int. J. Mol. Sci. 2022, 23, 7652. [Google Scholar] [CrossRef] [PubMed]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Wittaya-areekul, S.; Prahsarn, C. Development and in vitro evaluation of chitosan–polysaccharides composite wound dressings. Int. J. Pharm. 2006, 313, 123–128. [Google Scholar] [CrossRef]
- Phaechamud, T.; Issarayungyuen, P.; Pichayakorn, W. Gentamicin sulfate-loaded porous natural rubber films for wound dressing. Int. J. Biol. Macromol. 2016, 85, 634–644. [Google Scholar] [CrossRef]
- Williams, C.; Geroni, G.; Lloyd, D.; Choi, H.; Clark, N.; Pirog, A.; Lees, J.; Porch, A. Bioluminescence of Vibrio fischeri: Bacteria respond quickly and sensitively to pulsed microwave electric (but not magnetic) fields. J. Biomed. Opt. 2019, 24, 051412. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]















| Sample | Apparent Density (g/cm3) | Roughness Parameters (nm) | DPPH Scavenging (%) | ||
|---|---|---|---|---|---|
| Rq | Ra | Rmax | |||
| CS-Gel | 0.470 | 2.99 | 2.04 | 29.4 | 10.3 |
| CS-Gel-5%DNCL | 0.378 | 3.26 | 2.53 | 26.3 | 27.6 |
| CS-Gel-10%DNCL | 0.392 | 4.36 | 3.45 | 31.4 | 45.7 |
| CS-Gel-15%DNCL | 0.274 | 8.29 | 4.44 | 70.4 | 61.7 |
| CS-Gel-5%DAMC | 0.362 | 3.01 | 2.35 | 25.1 | 20.6 |
| CS-Gel-10%DAMC | 0.271 | 3.89 | 3.09 | 29.4 | 37.4 |
| CS-Gel-15%DAMC | 0.186 | 3.97 | 3.12 | 31.4 | 52.9 |
| Sample | WVTR (mg/cm2/h) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| CS-Gel | 1.03 ± 0.04 | 2.85 ± 0.07 | 4.90 ± 0.09 |
| CS-Gel-5%DNCL | 1.21 ± 0.02 | 3.50 ± 0.08 | 6.49 ± 0.21 |
| CS-Gel-10%DNCL | 1.44 ± 0.01 | 3.90 ± 0.03 | 8.60 ± 0.07 |
| CS-Gel-15%DNCL | 1.48 ± 0.11 | 4.19 ± 0.19 | 9.60 ± 0.06 |
| CS-Gel-5%DAMC | 1.12 ± 0.05 | 3.63 ± 0.05 | 5.24 ± 0.20 |
| CS-Gel-10%DAMC | 1.05 ± 0.03 | 3.60 ± 0.04 | 5.11 ± 0.20 |
| CS-Gel-15%DAMC | 1.13 ± 0.04 | 3.56 ± 0.02 | 6.34 ± 0.19 |
| Sample | Average Contact Angle (θ, °) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|
| Measuring Liquid | |||||
| Glycerin | Diiodomethane | γs | γsd | γsp | |
| CS-Gel | 71.6 | 46.8 | 36.70 | 30.76 | 5.95 |
| CS-Gel-5%DAMC | 74.0 | 44.3 | 37.35 | 32.91 | 4.44 |
| CS-Gel-10%DAMC | 69.4 | 43.6 | 38.52 | 32.14 | 6.38 |
| CS-Gel-15%DAMC | 69.8 | 45.2 | 37.78 | 31.28 | 6.50 |
| CS-Gel-5%DNCL | 66.8 | 45.6 | 38.42 | 30.30 | 8.12 |
| CS-Gel-10%DNCL | 68.1 | 43.7 | 38.80 | 31.75 | 7.04 |
| CS-Gel-15%DNCL | 67.9 | 45.4 | 38.18 | 30.69 | 7.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegrzynowska-Drzymalska, K.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Chitosan-Gelatin Films Cross-Linked with Dialdehyde Cellulose Nanocrystals as Potential Materials for Wound Dressings. Int. J. Mol. Sci. 2022, 23, 9700. https://doi.org/10.3390/ijms23179700
Wegrzynowska-Drzymalska K, Mlynarczyk DT, Chelminiak-Dudkiewicz D, Kaczmarek H, Goslinski T, Ziegler-Borowska M. Chitosan-Gelatin Films Cross-Linked with Dialdehyde Cellulose Nanocrystals as Potential Materials for Wound Dressings. International Journal of Molecular Sciences. 2022; 23(17):9700. https://doi.org/10.3390/ijms23179700
Chicago/Turabian StyleWegrzynowska-Drzymalska, Katarzyna, Dariusz T. Mlynarczyk, Dorota Chelminiak-Dudkiewicz, Halina Kaczmarek, Tomasz Goslinski, and Marta Ziegler-Borowska. 2022. "Chitosan-Gelatin Films Cross-Linked with Dialdehyde Cellulose Nanocrystals as Potential Materials for Wound Dressings" International Journal of Molecular Sciences 23, no. 17: 9700. https://doi.org/10.3390/ijms23179700