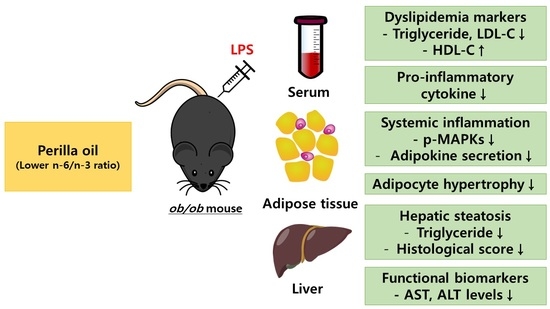
Lowering n-6/n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in ob/ob Mice
Abstract
:1. Introduction
2. Results
2.1. Dietary Fatty Acid Profiles in Experimental Diets
2.2. Effect of Partial Replacement of Dietary Fatty Acids with PUFAs on Body Weight (BW)
2.3. Alteration of Fatty Acids Composition after Dietary Intervention
2.4. Fatty Acids Replacement with PO or CO Maintains Intact Insulin Sensitivity in ob/ob Mice
2.5. Fatty Acids Replacement and LPS Injection did Not Alter Relative Tissue Weight
2.6. PO or CO Attenuates Cardiovascular Risk Markers in LPS-Injected ob/ob Mice
2.7. PO or CO Suppresses Systemic Inflammation in LPS-Injected ob/ob Mice
2.8. PO Alleviates Hepatic Lipid Accumulation and Fibrosis in LPS-Injected ob/ob Mice
2.9. PO Inhibits Lipid Accumulation and Inhibits Adipocyte Size, Leading to Suppression of Inflammation in EAT in Inflammatory ob/ob Mice
2.10. ER Stress and Oxidative Gene Expression in EAT from ob/ob Mice under Inflammation
3. Discussion
4. Materials and Methods
4.1. Animal Experiments and Diets
4.2. Analysis of Fatty Acids of Experimental Diets
4.3. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
4.4. Determination of Serum Metabolic Parameters
4.5. Analysis of the Fatty Acids Composition of Whole Blood
4.6. Measurement of Lipid Contents in the Liver and Adipose Tissue
4.7. Measurement of Adipokine Secretion Ex Vivo
4.8. Histological Evaluation of the Liver and Epididymal Adipose Tissue
4.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhurosy, T.; Jeewon, R. Overweight and obesity epidemic in developing countries: A problem with diet, physical activity, or socioeconomic status? Sci. World J. 2014, 2014, 964236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9 (Suppl. S4), 228S–233S. [Google Scholar] [CrossRef] [PubMed]
- Agha, M.; Agha, R. The rising prevalence of obesity: Part A: Impact on public health. Int. J. Surg. Oncol. 2017, 2, e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.C.; Cho, N.H.; Suh, S.; Kim, J.H.; Hur, K.Y.; Jin, S.M.; Lee, M.K. Cardiovascular disease incidence, mortality and case fatality related to diabetes and metabolic syndrome: A community-based prospective study (Ansung-Ansan cohort 2001-12). J. Diabetes 2015, 7, 791–799. [Google Scholar] [CrossRef]
- Daidj, N.B.B.; Lamri-Senhadji, M. Hepatoprotective and Anti-Obesity Properties of Sardine By-Product Oil in Rats Fed a High-Fat Diet. Prev. Nutr. Food Sci. 2021, 26, 285–295. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Verboven, K.; Wouters, K.; Gaens, K.; Hansen, D.; Bijnen, M.; Wetzels, S.; Stehouwer, C.D.; Goossens, G.H.; Schalkwijk, C.G.; Blaak, E.E.; et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci. Rep. 2018, 8, 4677. [Google Scholar] [CrossRef]
- Balkrishna, A.; Gohel, V.; Singh, R.; Joshi, M.; Varshney, Y.; Srivastava, J.; Bhattacharya, K.; Varshney, A. Tri-Herbal Medicine Divya Sarva-Kalp-Kwath (Livogrit) Regulates Fatty Acid-Induced Steatosis in Human HepG2 Cells through Inhibition of Intracellular Triglycerides and Extracellular Glycerol Levels. Molecules 2020, 25, 4849. [Google Scholar] [CrossRef]
- Hucik, B.; Lovell, A.J.; Hoecht, E.M.; Cervone, D.T.; Mutch, D.M.; Dyck, D.J. Regulation of adipose tissue lipolysis by ghrelin is impaired with high-fat diet feeding and is not restored with exercise. Adipocyte 2021, 10, 338–349. [Google Scholar] [CrossRef]
- Nogueira, S.; Garcez, F.; Sa, S.; Moutinho, L.C.; Cardoso, A.; Soares, R.; Fonseca, B.M.; Leal, S. Early unhealthy eating habits underlie morpho-functional changes in the liver and adipose tissue in male rats. Histochem. Cell Biol. 2022, 157, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Chen, Y.L.; Chiu, W.C.; Yeh, C.L.; Tung, Y.T.; Shirakawa, H.; Liao, W.T.; Yang, S.C. Effects of the Water Extract of Fermented Rice Bran on Liver Damage and Intestinal Injury in Aged Rats with High-Fat Diet Feeding. Plants 2022, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two ‘hits’? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Day, C.P. From fat to inflammation. Gastroenterology 2006, 130, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medeiros, G.; Azevedo, K.P.M.; Mesquita, G.X.B.; Lima, S.; Silva, D.F.O.; Pimenta, I.; Goncalves, A.; Lyra, C.O.; Piuvezam, G. Red meat consumption, risk of incidence of cardiovascular disease and cardiovascular mortality, and the dose-response effect: Protocol for a systematic review and meta-analysis of longitudinal cohort studies. Medicine 2019, 98, e17271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad Alinafiah, S.; Azlan, A.; Ismail, A.; Mahmud Ab Rashid, N.K. Method Development and Validation for Omega-3 Fatty Acids (DHA and EPA) in Fish Using Gas Chromatography with Flame Ionization Detection (GC-FID). Molecules 2021, 26, 6592. [Google Scholar] [CrossRef]
- Mendes Furtado, M.; Lima Rocha, J.E.; da Silva Mendes, A.V.; Mello Neto, R.S.; Brito, A.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa Franca, J.V.; Cunha Sales, A.L.C.; Gomes Vasconcelos, A.; et al. Effects of omega-3 PUFA-Rich Oil Supplementation on Cardiovascular Morphology and Aortic Vascular Reactivity of Adult Male Rats Submitted to an Hypercholesterolemic Diet. Biology 2022, 11, 202. [Google Scholar] [CrossRef]
- Kangwan, N.; Pratchayasakul, W.; Kongkaew, A.; Pintha, K.; Chattipakorn, N.; Chattipakorn, S.C. Perilla Seed Oil Alleviates Gut Dysbiosis, Intestinal Inflammation and Metabolic Disturbance in Obese-Insulin-Resistant Rats. Nutrients 2021, 13, 3141. [Google Scholar] [CrossRef]
- Cione, E.; Plastina, P.; Pingitore, A.; Perri, M.; Caroleo, M.C.; Fazio, A.; Witkamp, R.; Meijerink, J. Capsaicin Analogues Derived from n-3 Polyunsaturated Fatty Acids (PUFAs) Reduce Inflammatory Activity of Macrophages and Stimulate Insulin Secretion by beta-Cells In Vitro. Nutrients 2019, 11, 915. [Google Scholar] [CrossRef] [Green Version]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky, J., Jr.; Kudova, E.; et al. Docosahexaenoic Acid-Derived Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) with Anti-inflammatory Properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.J.; Arita, M.; Taguchi, R.; Kang, J.X.; Marette, A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes 2010, 59, 3066–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-alpha Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient. Pharm. Exp. Med. 2011, 11, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, N., Jr.; Litman, B.; Kim, H.Y.; Gawrisch, K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids 2001, 36, 945–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.H.; Hwang, H.J.; Shin, K.O.; Jeon, W.M.; Choi, K.S. Effects of perilla oil on plasma concentrations of cardioprotective (n-3) fatty acids and lipid profiles in mice. Nutr. Res. Pract. 2013, 7, 256–261. [Google Scholar] [CrossRef]
- Hashimoto, M.; Matsuzaki, K.; Kato, S.; Hossain, S.; Ohno, M.; Shido, O. Twelve-month Studies on Perilla Oil Intake in Japanese Adults-Possible Supplement for Mental Health. Foods 2020, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Jung, S.Y.; Park, Y.A.; Lee, Y.J.; Jo, J.Y.; Lee, S.M.; Oh, Y.H. Fatty acid composition and characterisation of commercial vegetable oils with chemometric approaches. Int. Food Res. J. 2020, 27, 270–279. [Google Scholar]
- Tortosa-Caparros, E.; Navas-Carrillo, D.; Marin, F.; Orenes-Pinero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, Y.; Yin, M.; Mao, L.; Zhang, S.; Pan, J. Low omega-6/omega-3 polyunsaturated fatty acid ratios reduce hepatic C-reactive protein expression in apolipoprotein E-null mice. Nutrition 2010, 26, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, H.K.; Kim, B.H.; Lee, J.; Park, S.; Oh, C.B.; Jung, S.; Lee, J.K.; Ha, J.H. Partial Replacement of Dietary Fat with Krill Oil or Coconut Oil Alleviates Dyslipidemia by Partly Modulating Lipid Metabolism in Lipopolysaccharide-Injected Rats on a High-Fat Diet. Int. J. Environ. Res. Public Health 2022, 19, 843. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.K.; Lee, J.-J.; Park, S.; Jung, S.; Lee, H.-J.; Ha, J.-H. Partial Replacement of High-fat Diet with Beef Tallow Attenuates Dyslipidemia and ER Stress in db/db Mice. J. Med. Food 2022. [Google Scholar] [CrossRef]
- Gao, S.; He, Y.; Zhang, L.; Liu, L.; Qu, C.; Zheng, Z.; Miao, J. Conjugated linoleic acid ameliorates hepatic steatosis by modulating intestinal permeability and gut microbiota in ob/ob mice. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Wade, J.M.; Juneja, P.; MacKay, A.W.; Graham, J.; Havel, P.J.; Tecott, L.H.; Goulding, E.H. Synergistic impairment of glucose homeostasis in ob/ob mice lacking functional serotonin 2C receptors. Endocrinology 2008, 149, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Faggioni, R.; Fantuzzi, G.; Gabay, C.; Moser, A.; Dinarello, C.A.; Feingold, K.R.; Grunfeld, C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am. J. Physiol. 1999, 276, R136–R142. [Google Scholar] [CrossRef]
- Son, H.K.; Xiang, H.; Park, S.; Lee, J.; Lee, J.J.; Jung, S.; Ha, J.H. Partial Replacement of Dietary Fat with Polyunsaturated Fatty Acids Attenuates the Lipopolysaccharide-Induced Hepatic Inflammation in Sprague-Dawley Rats Fed a High-Fat Diet. Int. J. Environ. Res. Public Health 2021, 18, 986. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; She, J.; Lin, F.; Wu, J.C.; Han, R.; Sheng, R.; Wang, G.; Qin, Z.H. RRx-001 Exerts Neuroprotection Against LPS-Induced Microglia Activation and Neuroinflammation Through Disturbing the TLR4 Pathway. Front. Pharmacol. 2022, 13, 889383. [Google Scholar] [CrossRef] [PubMed]
- Chenni, A.; Cherif, F.Z.H.; Chenni, K.; Elius, E.E.; Pucci, L.; Yahia, D.A. Effects of Pumpkin (Cucurbita pepo L.) Seed Protein on Blood Pressure, Plasma Lipids, Leptin, Adiponectin, and Oxidative Stress in Rats with Fructose-Induced Metabolic Syndrome. Prev. Nutr. Food Sci. 2022, 27, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim. Biophys. Acta 2014, 1837, 418–426. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Clement, K. Genetics of human obesity. C. R. Biol. 2006, 329, 608–622. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [Green Version]
- Manjunath, C.N.; Rawal, J.R.; Irani, P.M.; Madhu, K. Atherogenic dyslipidemia. Indian J. Endocrinol. Metab. 2013, 17, 969–976. [Google Scholar] [CrossRef]
- Hasheminasabgorji, E.; Jha, J.C. Dyslipidemia, Diabetes and Atherosclerosis: Role of Inflammation and ROS-Redox-Sensitive Factors. Biomedicines 2021, 9, 1602. [Google Scholar] [CrossRef]
- Lechner, K.; McKenzie, A.L.; Krankel, N.; Von Schacky, C.; Worm, N.; Nixdorff, U.; Lechner, B.; Scherr, J.; Weingartner, O.; Krauss, R.M. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, Y.K.; Li, J.; Yi, L.; Zhu, R.L.; Luo, J.F.; Shi, Q.P.; Bai, S.S.; Li, Y.W.; Du, Q.; Cai, J.Z.; et al. Sinomenine inhibits macrophage M1 polarization by downregulating alpha7nAChR via a feedback pathway of alpha7nAChR/ERK/Egr-1. Phytomedicine 2022, 100, 154050. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, K.F.; Almalki, A.A.; Alsanie, W.F.; Alzahrani, K.J.; Kabrah, S.M.; Elshopakey, G.E.; Alghamdi, A.A.A.; Lokman, M.S.; Sberi, H.A.; Bauomy, A.A.; et al. Protocatechuic acid attenuates lipopolysaccharide-induced septic lung injury in mice: The possible role through suppressing oxidative stress, inflammation and apoptosis. J. Food Biochem. 2021, 45, e13915. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hong, H.L.; Kim, G.M.; Leem, J.; Kwon, H.H. Protective Effects of Carnosic Acid on Lipopolysaccharide-Induced Acute Kidney Injury in Mice. Molecules 2021, 26, 7589. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, S.; Shang, F.; Ning, Y.; Huang, Z.; He, R.; Sun, J.; Dong, S. Emodin Improves Glucose and Lipid Metabolism Disorders in Obese Mice via Activating Brown Adipose Tissue and Inducing Browning of White Adipose Tissue. Front. Endocrinol. 2021, 12, 618037. [Google Scholar] [CrossRef]
- Olson, N.C.; Callas, P.W.; Hanley, A.J.; Festa, A.; Haffner, S.M.; Wagenknecht, L.E.; Tracy, R.P. Circulating levels of TNF-alpha are associated with impaired glucose tolerance, increased insulin resistance, and ethnicity: The Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 2012, 97, 1032–1040. [Google Scholar] [CrossRef]
- Qu, D.; Liu, J.; Lau, C.W.; Huang, Y. IL-6 in diabetes and cardiovascular complications. Br. J. Pharmacol. 2014, 171, 3595–3603. [Google Scholar] [CrossRef] [Green Version]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef]
- Butterfield, T.A.; Best, T.M.; Merrick, M.A. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J. Athl. Train. 2006, 41, 457–465. [Google Scholar]
- Chen, L.Z.; Jing, X.B.; Wu, C.F.; Zeng, Y.C.; Xie, Y.C.; Wang, M.Q.; Chen, W.X.; Hu, X.; Zhou, Y.N.; Cai, X.B. Nonalcoholic Fatty Liver Disease-Associated Liver Fibrosis Is Linked with the Severity of Coronary Artery Disease Mediated by Systemic Inflammation. Dis. Markers 2021, 2021, 6591784. [Google Scholar] [CrossRef]
- Lima Rocha, J.E.; Mendes Furtado, M.; Mello Neto, R.S.; da Silva Mendes, A.V.; Brito, A.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa Franca, J.V.; Silva Primo, M.G.; Cunha Sales, A.L.C.; et al. Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients 2022, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P. Pathology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. S1), 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Araya, J.; Rodrigo, R.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Bogl, L.H.; Kaprio, J.; Pietilainen, K.H. Dietary n-6 to n-3 fatty acid ratio is related to liver fat content independent of genetic effects: Evidence from the monozygotic co-twin control design. Clin. Nutr. 2020, 39, 2311–2314. [Google Scholar] [CrossRef]
- Ma, L.; Wu, F.; Shao, Q.; Chen, G.; Xu, L.; Lu, F. Baicalin Alleviates Oxidative Stress and Inflammation in Diabetic Nephropathy via Nrf2 and MAPK Signaling Pathway. Drug Des. Devel. Ther. 2021, 15, 3207–3221. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Fernandez-Checa, J.C.; Kaplowitz, N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014, 5, e989. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C.S. Artemisia princeps Inhibits Adipogenic Differentiation of 3T3-L1 Pre-Adipocytes via Downregulation of PPARgamma and MAPK Pathways. Prev. Nutr. Food Sci. 2019, 24, 299–307. [Google Scholar] [CrossRef]
- Sharma, D.; Nkembi, A.S.; Aubry, E.; Houeijeh, A.; Butruille, L.; Houfflin-Debarge, V.; Besson, R.; Deruelle, P.; Storme, L. Maternal PUFA omega-3 Supplementation Prevents Neonatal Lung Injuries Induced by Hyperoxia in Newborn Rats. Int. J. Mol. Sci. 2015, 16, 22081–22093. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Ping, F.; Cao, Y.; Shang, J.; Zhang, J.; Jiang, H.; Fang, S. Effects of Omega-3 Polyunsaturated Fatty Acids on Cognitive Function after Splenectomy in Rats. Biomed. Res. Int. 2021, 2021, 5513886. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, L.; Zhou, Z.; Song, L.; Chen, S.; Yang, Y.; Hu, Y.; Wang, Y.; Wu, S.; Tian, Y. Lifetime risk of cardiovascular disease and life expectancy with and without cardiovascular disease according to changes in metabolic syndrome status. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Inoue, S.; Taniguchi, Y.; Miyahara, H.; Iwasaki, Y.; Takeo, J.; Sakaue, H.; Nakaya, Y. Long-term dietary supplementation with saury oil attenuates metabolic abnormalities in mice fed a high-fat diet: Combined beneficial effect of omega-3 fatty acids and long-chain monounsaturated fatty acids. Lipids Health Dis. 2015, 14, 155. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health and Welfare; The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2020; The Korean Nutrition Society: Sejong, Korea, 2020. [Google Scholar]
- Medicine, I.O. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies Press: Washington, DC, USA, 2006; p. 1344. [Google Scholar]
- Committee, E.S.; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hougaard Bennekou, S.; Koutsoumanis, K.; Machera, K.; Naegeli, H.; et al. Statement on the derivation of Health-Based Guidance Values (HBGVs) for regulated products that are also nutrients. EFSA J. 2021, 19, e06479. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Chinese Dietary Reference Intakes—Part 1: Macronutrient; Chinese Nutrition Society: Beijing, China, 2017. [Google Scholar]
- Park, S.; Lee, J.J.; Shin, H.W.; Jung, S.; Ha, J.H. Effect of Soybean and Soybean Koji on Obesity and Dyslipidemia in Rats Fed a High-Fat Diet: A Comparative Study. Int. J. Environ. Res. Public Health 2021, 18, 6032. [Google Scholar] [CrossRef]
- Moyers, B.; Farzaneh-Far, R.; Harris, W.S.; Garg, S.; Na, B.; Whooley, M.A. Relation of whole blood n-3 fatty acid levels to exercise parameters in patients with stable coronary artery disease (from the heart and soul study). Am. J. Cardiol. 2011, 107, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Park, Y.H.; Lee, J.J.; Son, H.K.; Kim, B.H.; Byun, J.; Ha, J.H. Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients 2020, 12, 336. [Google Scholar] [CrossRef] [Green Version]


















| ND (g) | PO (g) | CO (g) | |
|---|---|---|---|
| Casein | 200 | 200 | 200 |
| L-cysteine | 3 | 3 | 3 |
| Sucrose | 100 | 100 | 100 |
| Cornstarch | 397.5 | 397.5 | 397.5 |
| Dextrose | 132 | 132 | 132 |
| tert-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Cellulose | 50 | 50 | 50 |
| Mineral mix | 35 | 35 | 35 |
| Vitamin mix | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Western blend | 50 | 45 | 30 |
| Lard | 20 | 15 | 10 |
| PO | - | 10 | - |
| CO | - | - | 30 |
| Total (g) | 1000.014 | 1000.014 | 1000.014 |
| Total energy (kcal/g) | 4000 | 4000 | 4000 |
| Energy from fat (kcal%) | 15.75 | 15.75 | 15.75 |
| ND (%) | PO (%) | CO (%) | |
|---|---|---|---|
| Palmitic acid (C16:0) | 37.61 ± 0.93 a | 33.86 ± 2.60 b | 33.26 ± 2.11 b |
| Stearic acid (C18:0) | 15.46 ± 3.58 a | 10.91 ± 0.75 b | 8.78 ± 0.61 b |
| Elaidic acid (C18:1n-9t) | 4.23 ± 0.94 a | 4.18 ± 0.12 a | 1.76 ± 0.10 b |
| Oleic acid (C18:1n-9c) | 40.30 ± 1.39 a | 36.61 ± 1.00 b | 24.66 ± 1.48 c |
| Linoleic acid (C18:2n-6c) | 0.75 ± 0.44 c | 5.03 ± 0.76 b | 21.51 ± 0.14 a |
| α-linolenic acid (C18:3n-3) | 0.82 ± 0.20 b | 10.44 ± 0.89 a | 10.02 ± 0.72 a |
| SFA (%) | 53.07 | 44.77 | 42.04 |
| MUFA (%) | 44.78 | 40.79 | 26.42 |
| PUFA (%) | 2.02 | 15.47 | 31.53 |
| n-3 (%) | 1.52 | 10.44 | 10.02 |
| n-6 (%) | 0.75 | 5.03 | 21.51 |
| Total fatty acid (%) | 100.00 | 100.00 | 100.00 |
| ND (%) | PO (%) | CO (%) | |
|---|---|---|---|
| α-linolenic acid | 0.11 ± 0.07 b | 0.3 ± 0.05 a | 0.04 ± 0.02 c |
| Eicosapentaenoic acid (EPA) | 0.27 ± 0.12 b | 3.57 ± 0.36 a | 0.35 ± 0.09 b |
| Docosapentaenoic acid (DPA) | 0.24 ± 0.10 b | 1.42 ± 0.15 a | 0.26 ± 0.05 b |
| Docosahexaenoic acid (DHA) | 2.43 ± 1.97 c | 8.12 ± 0.41 a | 4.76 ± 0.42 b |
| Linoleic acid | 6.09 ± 0.91 c | 7.06 ± 0.23 b | 8.07 ± 0.95 a |
| γ-linolenic acid | 0.04 ± 0.01 b | 0.04 ± 0.02 b | 0.06 ± 0.01 a |
| Eicosadienoic acid | 0.22 ± 0.21 a | 0.14 ± 0.04 a | 0.15 ± 0.03 a |
| Dihomo-γ-linolenic acid | 1.36 ± 0.56 a | 1.42 ± 0.07 a | 1.56 ± 0.17 a |
| Arachidonic acid | 12.41 ± 4.81 b | 10.20 ± 0.27 b | 20.20 ± 1.71 a |
| Docosatetraenoic acid | 0.55 ± 0.19 b | 0.33 ± 0.02 c | 1.06 ± 0.10 a |
| Docosapentaenoic acid | 0.42 ± 0.17 b | 0.08 ± 0.05 c | 0.67 ± 0.10 a |
| Oleic acid | 30.15 ± 7.11 a | 23.55 ± 0.90 b | 19.45 ± 2.24 b |
| Eicosenoic acid | 0.32 ± 0.03 a | 0.24 ± 0.02 b | 0.24 ± 0.01 b |
| Nervonic acid | 0.06 ± 0.03 a | 0.08 ± 0.02 a | 0.05 ± 0.01 a |
| Palmitoleic acid | 7.44 ± 3.98 a | 3.88 ± 0.70 b | 3.29 ± 0.92 b |
| Myristic acid | 1.36 ± 0.83 a | 0.70 ± 0.20 b | 0.66 ± 0.38 b |
| Palmitic acid | 25.73 ± 1.29 c | 28.50 ± 0.85 a | 27.10 ± 1.03 b |
| Stearic acid | 11.88 ± 1.96 a | 9.67 ± 0.10 b | 11.15 ± 1.22 a |
| Lignoceric acid | 0.20 ± 0.07 a | 0.17 ± 0.05 a | 0.20 ± 0.03 a |
| n-3 | 2.05 ± 2.10 b | 13.41 ± 0.60 a | 5.40 ± 0.47 b |
| n-6 | 21.09 ± 6.71 b | 19.27 ± 0.35 b | 31.77 ± 1.40 a |
| n-9 | 30.53 ± 7.12 a | 23.86 ± 0.89 b | 19.76 ± 2.25 b |
| SFA | 39.16 ± 2.40 b | 39.03 ± 0.77 a | 39.11 ± 1.67 a |
| MUFA | 37.97 ± 11.10 a | 27.74 ± 0.42 b | 23.03 ± 3.14 b |
| n-6/n-3 | 10.30 ± 0.48 a | 1.45 ± 0.07 c | 5.92 ± 0.33 b |
| AA/EPA | 48.13 ± 0.48 b | 2.9 ± 0.42 c | 60.98 ± 1.67 a |
| Authority | Dietary Fat | Recommendation |
|---|---|---|
| fat | 25–35% | |
| American Heart Association (AHA) | n-3 fatty acids | - Consumption of fish or shellfish 1–2 times per week (250 mg/day calculated with EPA + DHA level) - Daily intake of EPA + DHA for healthy people (500 mg/day) and for CVD patient (800–1000 mg/day) |
| U.S. | fat linoleic acid alpha-linolenic acid (ALA) n-3 fatty acids | 20–35% 11–17 g/day 1.1–1.6 g/day 0.6–1.2% of total energy intake |
| Europe | Fat | Less than 30% |
| SFA less than 10% | ||
| Trans fat less than 1% | ||
| FAO | PUFAs | 6–10 % of total energy intake |
| n-3 fatty acids | 1–2% | |
| n-6 fatty acids | 5–8% | |
| Institute of Medicine (IOM) | n-3 fatty acids | ~1.6 g/day |
| n-6 fatty acids | ~17 g/day |
| Transcript | Forward | Reverse |
|---|---|---|
| Pparγ | GGC GAT CTT GAC AGG AAA GAC | CCC TTG AAA AAT TCG GAT GG |
| Cebpα | GGT TTT GCT CTG ATT CTT GCC | CGA AAA AAC CCA AAC ATC CC |
| aP2 | AGC ATC ATA ACC CTA GAT GGC G | CAT AAC ACA TTC CAC CAC CAG C |
| Pgc1α | CCC TGC CAT TGT TAA GAC C | TGC TGC TGT TCC TGT TTT C |
| Il-6 | CTG CAA GAG ACT TCC ATC CAG TT | AGG GAA GGC CGT GGT TGT |
| Il-1β | GTC ACA AGA AAC CAT GGC ACA T | GCC CAT CAG AGG CAA GGA |
| Gapdh | CAT GGC CTT CCG TGT TCC TA | GCG GCA CGT CAG ATC CA |
| Antibody | Dilution Factor | Corporation | Catalog Number | |
|---|---|---|---|---|
| Primary antibody | p-JNK | 1:500 | Cell Signaling | 9251 |
| p-ERK | 1:3000 | Cell Signaling | 4370 | |
| BiP | 1:1000 | Cell Signaling | 3183 | |
| CHOP | 1:1000 | Cell Signaling | 2895 | |
| HO-1 | 1:1000 | Cell Signaling | 5853S | |
| β-actin | 1:2000 | Santa Cruz | sc-47778 | |
| Secondary antibody | Anti-rabbit IgG | 1:3000 | Cell Signaling | 7074 |
| Anti-mouse IgG | 1:1000 | Cell Signaling | 7076 |
| Parameter | LPS Main Effect | Diet Main Effect | LPS × Diet Interaction |
|---|---|---|---|
| Relative tissue weights | |||
| Liver | ns p = 0.315 | ns p = 0.422 | ns p = 0.616 |
| Epididymal adipose tissue | ns p = 0.940 | ns p = 0.170 | ns p = 0.783 |
| Mesenteric adipose tissue | ns p = 0.497 | ns p = 0.877 | ns p = 0.951 |
| Retroperitoneal adipose tissue | ns p = 0.214 | ns p = 0.359 | ns p = 0.537 |
| Perirenal adipose tissue | *** p < 0.001 | ns p = 0.591 | ns p = 0.777 |
| White adipose tissue | ns p = 0.630 | ns p = 0.092 | ns p = 0.555 |
| Serum lipid profiles | |||
| Triglyceride | **** p < 0.0001 | ns p = 0.795 | ns p = 0.732 |
| Total cholesterol | **** p < 0.0001 | ns p = 0.292 | * p < 0.05 |
| HDL-cholesterol | **** p < 0.0001 | ** p < 0.01 | * p < 0.05 |
| LDL-cholesterol | **** p < 0.0001 | * p < 0.05 | ** p < 0.01 |
| Cardiac risk factor | **** p < 0.0001 | *** p < 0.001 | *** p < 0.001 |
| Serum inflammatory mediators | |||
| IL-1β | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 |
| IL-10 | **** p < 0.0001 | **** p < 0.0001 | **** p < 0.0001 |
| TNF-α | ** p < 0.01 | *** p < 0.001 | * p < 0.05 |
| CXCL-1 | **** p < 0.0001 | * p < 0.05 | * p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lee, J.-J.; Lee, J.; Lee, J.K.; Byun, J.; Kim, I.; Ha, J.-H. Lowering n-6/n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in ob/ob Mice. Int. J. Mol. Sci. 2022, 23, 6384. https://doi.org/10.3390/ijms23126384
Park S, Lee J-J, Lee J, Lee JK, Byun J, Kim I, Ha J-H. Lowering n-6/n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in ob/ob Mice. International Journal of Molecular Sciences. 2022; 23(12):6384. https://doi.org/10.3390/ijms23126384
Chicago/Turabian StylePark, Seohyun, Jae-Joon Lee, Jisu Lee, Jennifer K. Lee, Jaemin Byun, Inyong Kim, and Jung-Heun Ha. 2022. "Lowering n-6/n-3 Ratio as an Important Dietary Intervention to Prevent LPS-Inducible Dyslipidemia and Hepatic Abnormalities in ob/ob Mice" International Journal of Molecular Sciences 23, no. 12: 6384. https://doi.org/10.3390/ijms23126384