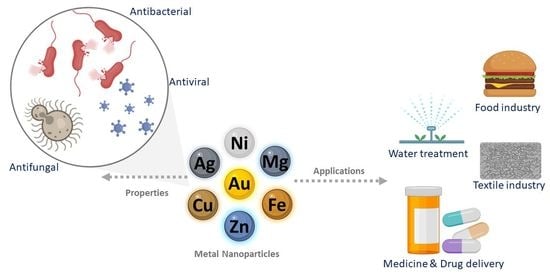
Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms
Abstract
:1. Introduction
2. Mechanisms behind the Antimicrobial Effect
3. Metallic Particles—Nano- and Microstructures
3.1. Nanoparticles
3.2. Microparticles
4. Antimicrobial Surfaces and Coatings
5. Synergic Combination with Drugs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selvamani, V.; Zareei, A.; Elkashif, A.; Maruthamuthu, M.K.; Chittiboyina, S.; Delisi, D.; Li, Z.; Cai, L.; Pol, V.G.; Seleem, M.N.; et al. Hierarchical Micro/Mesoporous Copper Structure with Enhanced Antimicrobial Property via Laser Surface Texturing. Adv. Mater. Interfaces 2020, 7, 1901890. [Google Scholar] [CrossRef]
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-Gum Arabic Embedded Alizarin Nanocarriers Inhibit Biofilm Formation of Multispecies Microorganisms. Carbohydr. Polym. 2021, 2021, 118959. [Google Scholar] [CrossRef]
- Ghosh, M.; Mandal, S.; Roy, A.; Chakrabarty, S.; Chakrabarti, G.; Pradhan, S.K. Enhanced antifungal activity of fluconazole conjugated with Cu-Ag-ZnO nanocomposite. Mater. Sci. Eng. C 2020, 106, 110160. [Google Scholar] [CrossRef] [PubMed]
- Kefallinou, D.; Ellinas, K.; Speliotis, T.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Optimization of Antibacterial Properties of “Hybrid” Metal-Sputtered Superhydrophobic Surfaces. Coatings 2019, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.M.; Keller, A. DNA Nanostructures in the Fight Against Infectious Diseases. Adv. NanoBiomed Res. 2021, 1, 2000049. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved a-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB 2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Raj, V.; Park, J.G.; Cho, K.H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Sheldon, J.R.; Skaar, E.P. Metals as phagocyte antimicrobial effectors. Curr. Opin. Immunol. 2019, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Agatemor, C.; Etkin, N. Antimicrobial resistance challenged with metal-based antimicrobial macromolecules. Biomaterials 2017, 118, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.K.; Kumar, K.S.; Parthiban, R.; Kalishwaralal, K. An insight study on HPTLC fingerprinting of Mukia maderaspatna: Mechanism of bioactive constituents in metal nanoparticle synthesis and its activity against human pathogens. Microb. Pathog. 2017, 102, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Applications, T. Springer Handbook of Petroleum Technology; Hsu, C.S., Robinson, P.R., Eds.; Springer Handbooks; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-49345-9. [Google Scholar]
- Akbarzadeh, A.; Kafshdooz, L.; Razban, Z.; Dastranj Tbrizi, A.; Rasoulpour, S.; Khalilov, R.; Kavetskyy, T.; Saghfi, S.; Nasibova, A.N.; Kaamyabi, S.; et al. An overview application of silver nanoparticles in inhibition of herpes simplex virus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 263–267. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Fernando, S.; Gunasekara, T.; Holton, J. Antimicrobial Nanoparticles: Applications and mechanisms of action. Sri Lankan J. Infect. Dis. 2018, 8, 2. [Google Scholar] [CrossRef]
- Crane, J.K. Metal Nanoparticles in Infection and Immunity. Immunol. Investig. 2020, 49, 794–807. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Virji, M.A.; Stefaniak, A.B. A Review of Engineered Nanomaterial Manufacturing Processes and Associated Exposures; Elsevier: Amsterdam, The Netherlands, 2014; Volume 8, ISBN 9780080965338. [Google Scholar]
- Neikov, O.D.; Naboychenko, S.S.; Murashova, I.B. Production of Noble Metal Powders, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081005439. [Google Scholar]
- Mensah, E.E.; Abbas, Z.; Azis, R.S.; Khamis, A.M. Enhancement of Complex Permittivity and Attenuation Properties of Recycled Hematite (α-Fe2O3) Using Nanoparticles Prepared via Ball Milling Technique. Materials 2019, 12, 1696. [Google Scholar] [CrossRef] [Green Version]
- Karthik, S.; Suriyaprabha, R.; Balu, K.S.; Manivasakan, P.; Rajendran, V. Influence of ball milling on the particle size and antimicrobial properties of Tridax procumbens leaf nanoparticles. IET Nanobiotechnol. 2017, 11, 12–17. [Google Scholar] [CrossRef]
- Alshora, D.H.; Ibrahim, M.A.; Alanazi, F.K. Nanotechnology from particle size reduction to enhancing aqueous solubility. In Surface Chemistry of Nanobiomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 163–191. ISBN 9780323428613. [Google Scholar]
- Singh, J.; Sharma, S.; Soni, S.; Sharma, S.; Chand Singh, R. Influence of different milling media on structural, morphological and optical properties of the ZnO nanoparticles synthesized by ball milling process. Mater. Sci. Semicond. Process. 2019, 98, 29–38. [Google Scholar] [CrossRef]
- Ayuk, E.L.; Ugwu, M.O.; Aronimo, S.B. A Review on Synthetic Methods of Nanostructured. Chem. Res. J. 2017, 2, 97–123. [Google Scholar]
- Cheng, W.; Zhang, W.; Hu, L.; Ding, W.; Wu, F.; Li, J. Etching synthesis of iron oxide nanoparticles for adsorption of arsenic from water. RSC Adv. 2016, 6, 15900–15910. [Google Scholar] [CrossRef]
- Verma, M.; Tyagi, I.; Chandra, R.; Gupta, V.K. Adsorptive removal of Pb (II) ions from aqueous solution using CuO nanoparticles synthesized by sputtering method. J. Mol. Liq. 2017, 225, 936–944. [Google Scholar] [CrossRef]
- Strijckmans, K.; Schelfhout, R.; Depla, D. Tutorial: Hysteresis during the reactive magnetron sputtering process. J. Appl. Phys. 2018, 124, 241101. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Boutinguiza, M.; del Val, J.; Riveiro, A.; Rodríguez, D.; Arias-González, F.; Gil, J.; Pou, J. Fabrication and Deposition of Copper and Copper Oxide Nanoparticles by Laser Ablation in Open Air. Nanomaterials 2020, 10, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, W.-H.; Hwang, Y.-T.; Lee, S.-H.; Kim, H.-S. Electrical wire explosion process of copper/silver hybrid nano-particle ink and its sintering via flash white light to achieve high electrical conductivity. Nanotechnology 2016, 27, 205704. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, A.; Ahmadi, R.; Yarali, D.; Sanaei, N. Synthesis of MoO2 nanoparticles via the electro-explosion of wire (EEW) method. Mater. Res. Express 2020, 6, 1250d3. [Google Scholar] [CrossRef]
- de Leon, A.; Advincula, R.C. Conducting Polymers with Superhydrophobic Effects as Anticorrosion Coating. In Intelligent Coatings for Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2015; pp. 409–430. ISBN 9780124115347. [Google Scholar]
- A C Sequeira, C. Electrochemical Synthesis of Iron Oxide Nanoparticles for Biomedical Application. Org. Med. Chem. Int. J. 2018, 5, 555660. [Google Scholar] [CrossRef]
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. Electrochemical method for the synthesis of silver nanoparticles. J. Nanopart. Res. 2009, 11, 1193–1200. [Google Scholar] [CrossRef]
- Anu Mary Ealia, S.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Madhukar, M.; Das, P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Bankier, C.; Matharu, R.K.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [Green Version]
- Singh, L.; Kruger, H.G.; Maguire, G.E.M.; Govender, T.; Parboosing, R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017, 4, 105–131. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Conte-Junior, C.A. Recent Advances on Nanomaterials to COVID-19 Management: A Systematic Review on Antiviral/Virucidal Agents and Mechanisms of SARS-CoV-2 Inhibition/Inactivation. Glob. Chall. 2021, 5, 2000115. [Google Scholar] [CrossRef]
- Khurana, C.; Sharma, P.; Pandey, O.P.; Chudasama, B. Synergistic Effect of Metal Nanoparticles on the Antimicrobial Activities of Antibiotics against Biorecycling Microbes. J. Mater. Sci. Technol. 2016, 32, 524–532. [Google Scholar] [CrossRef]
- Nguyen, N.-Y.T.; Grelling, N.; Wetteland, C.L.; Rosario, R.; Liu, H. Antimicrobial Activities and Mechanisms of Magnesium Oxide Nanoparticles (nMgO) against Pathogenic Bacteria, Yeasts, and Biofilms. Sci. Rep. 2018, 8, 16260. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Rhim, J.-W. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim. Biophys. Acta—Gen. Subj. 2015, 1850, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.O.; Ajayi, S.O.; Alausa, A.O.; Ogundile, O.P.; Ademosun, O.T. Antimicrobial and antibiofilm activities of green synthesized silver nanoparticles for water treatment. J. Phys. Conf. Ser. 2021, 1734, 012043. [Google Scholar] [CrossRef]
- de Toledo, L.d.A.S.; Rosseto, H.C.; Bruschi, M.L. Iron oxide magnetic nanoparticles as antimicrobials for therapeutics. Pharm. Dev. Technol. 2018, 23, 316–323. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Yamayoshi, I.; Mathew, S.; Lin, H.; Nayfach, J.; Simon, S.I. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann. Biomed. Eng. 2013, 41, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Giri, V.P.; Pandey, S.; Kumar, M.; Katiyar, R.; Nautiyal, C.S.; Mishra, A. An insight into the mechanism of antifungal activity of biogenic nanoparticles than their chemical counterparts. Pestic. Biochem. Physiol. 2019, 157, 45–52. [Google Scholar] [CrossRef]
- Li, J.; Sang, H.; Guo, H.; Popko, J.T.; He, L.; White, J.C.; Parkash Dhankher, O.; Jung, G.; Xing, B. Antifungal mechanisms of ZnO and Ag nanoparticles to Sclerotinia homoeocarpa. Nanotechnology 2017, 28, 155101. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X.; Pentok, M.; Sauli, E.; He, N.; Zen, X.; Li, X.; Liu, T. Molecular Mechanism and Changes of Antioxidant Enzyme in ZnO Nanoparticles Against Fungus. J. Biomed. Nanotechnol. 2019, 15, 647–661. [Google Scholar] [CrossRef]
- Mahdavi, B.; Saneei, S.; Qorbani, M.; Zhaleh, M.; Zangeneh, A.; Zangeneh, M.M.; Pirabbasi, E.; Abbasi, N.; Ghaneialvar, H. Ziziphora clinopodioides Lam leaves aqueous extract mediated synthesis of zinc nanoparticles and their antibacterial, antifungal, cytotoxicity, antioxidant, and cutaneous wound healing properties under in vitro and in vivo conditions. Appl. Organomet. Chem. 2019, 33, e5164. [Google Scholar] [CrossRef]
- Punjabi, K.; Mehta, S.; Chavan, R.; Chitalia, V.; Deogharkar, D.; Deshpande, S. Efficiency of Biosynthesized Silver and Zinc Nanoparticles Against Multi-Drug Resistant Pathogens. Front. Microbiol. 2018, 9, 2207. [Google Scholar] [CrossRef] [Green Version]
- Attia, G.H.; Moemen, Y.S.; Youns, M.; Ibrahim, A.M.; Abdou, R.; El Raey, M.A. Antiviral zinc oxide nanoparticles mediated by hesperidin and in silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B Biointerfaces 2021, 203, 111724. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Mahdinejad, N.; Ebrahimy, O.; Khatami, M.; Sarani, M. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater. Sci. Eng. C 2019, 104, 109981. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef]
- Sedaghat Anbouhi, T.; Mokhtari Esfidvajani, E.; Nemati, F.; Haghighat, S.; Sari, S.; Attar, F.; Pakaghideh, A.; Sohrabi, M.J.; Mousavi, S.E.; Falahati, M. Albumin binding, anticancer and antibacterial properties of synthesized zero valent iron nanoparticles. Int. J. Nanomed. 2018, 14, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Kumar, R.; Dalal, U.; Tomar, S.; Reddy, S.N. Green synthesis of nanometal impregnated biomass—Antiviral potential. Mater. Sci. Eng. C 2020, 112, 110934. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Khan, S.; Bo, B.; Zada, S.; Fu, P.C. Green synthesis and characterization of Fe3O4 nanoparticles using Chlorella-K01 extract for potential enhancement of plant growth stimulating and antifungal activity. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Shah, M.A.; Devi, H.S.; Bhat, M.Y.; Koka, J.A. Preparation, characterization and antifungal activity of iron oxide nanoparticles. Microb. Pathog. 2018, 115, 287–292. [Google Scholar] [CrossRef]
- Seydi, N.; Mahdavi, B.; Paydarfard, S.; Zangeneh, A.; Zangeneh, M.M.; Najafi, F.; Jalalvand, A.R.; Pirabbasi, E. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides Lam leaves. Appl. Organomet. Chem. 2019, 33, e5009. [Google Scholar] [CrossRef]
- Arya, S.; Sonawane, H.; Math, S.; Tambade, P.; Chaskar, M.; Shinde, D. Biogenic titanium nanoparticles (TiO2NPs) from Tricoderma citrinoviride extract: Synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int. Nano Lett. 2021, 11, 35–42. [Google Scholar] [CrossRef]
- Noori, A.J.; Kareem, F.A. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon 2019, 5, e02568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Rafiei, S.; Rezatofighi, S.E.; Ardakani, M.R.; Madadgar, O. In vitro anti-foot-and-mouth disease virus activity of magnesium oxide nanoparticles. IET Nanobiotechnol. 2015, 9, 247–251. [Google Scholar] [CrossRef]
- Hassanien, R.; Husein, D.Z.; Al-Hakkani, M.F. Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon 2018, 4, e01077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiriyanthan, R.M.; Sharmili, S.A.; Balaji, R.; Jayashree, S.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Govindarajan, M.; Vaseeharan, B. Photocatalytic, antiproliferative and antimicrobial properties of copper nanoparticles synthesized using Manilkara zapota leaf extract: A photodynamic approach. Photo Diagn. Photodyn. Ther. 2020, 32, 102058. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Etemadifar, Z.; Daneshkazemi, A.; Nateghi, M. Antimicrobial Effect of Copper Oxide Nanoparticles on Some Oral Bacteria and Candida Species. J. Dent. Biomater. 2017, 4, 347–352. [Google Scholar]
- Tavakoli, A.; Hashemzadeh, M.S. Inhibition of herpes simplex virus type 1 by copper oxide nanoparticles. J. Virol. Methods 2020, 275, 113688. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M.N. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci. Rep. 2020, 10, 16680. [Google Scholar] [CrossRef]
- Donga, S.; Bhadu, G.R.; Chanda, S. Antimicrobial, antioxidant and anticancer activities of gold nanoparticles green synthesized using Mangifera indica seed aqueous extract. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Joseph, S.; Mathew, B. Eco-friendly synthesis of silver and gold nanoparticles with enhanced antimicrobial, antioxidant, and catalytic activities. IET Nanobiotechnol. 2018, 12, 850–856. [Google Scholar] [CrossRef]
- Kim, J.; Yeom, M.; Lee, T.; Kim, H.-O.; Na, W.; Kang, A.; Lim, J.-W.; Park, G.; Park, C.; Song, D.; et al. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020, 18, 54. [Google Scholar] [CrossRef] [Green Version]
- Halder, A.; Das, S.; Ojha, D.; Chattopadhyay, D.; Mukherjee, A. Highly monodispersed gold nanoparticles synthesis and inhibition of herpes simplex virus infections. Mater. Sci. Eng. C 2018, 89, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT 2019, 103, 293–300. [Google Scholar] [CrossRef]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.-K.M.; Kaviyarasu, K.; Ravindran, B.; Chang, S.W.; Arokiyaraj, S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B Biol. 2019, 191, 65–74. [Google Scholar] [CrossRef]
- Jebril, S.; Khanfir Ben Jenana, R.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef] [Green Version]
- Haggag, E.; Elshamy, A.; Rabeh, M.; Gabr, N.; Salem, M.; Youssif, K.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef] [Green Version]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, T.V.M.; Pandurangan, M.; Kim, D.H.; Lee, Y.R. Green Synthesis: In-vitro Anticancer Activity of Silver Nanoparticles on Human Cervical Cancer Cells. J. Clust. Sci. 2016, 27, 671–681. [Google Scholar] [CrossRef]
- Aderibigbe, B. Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22, 1370. [Google Scholar] [CrossRef]
- Maduray, K.; Parboosing, R. Metal Nanoparticles: A Promising Treatment for Viral and Arboviral Infections. Biol. Trace Elem. Res. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral Potential of Nanoparticles—Can Nanoparticles Fight Against Coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Stack, M.; Parikh, D.; Wang, H.; Wang, L.; Xu, M.; Zou, J.; Cheng, J.; Wang, H. Electrospun Nanofibers for Drug Delivery. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 735–764. ISBN 9780323512701. [Google Scholar]
- Willerth, S. Drug delivery systems for engineering neural tissue. In Engineering Neural Tissue from Stem Cells; Elsevier: Amsterdam, The Netherlands, 2017; pp. 159–180. ISBN 9780128113851. [Google Scholar]
- Jin, S.-E.; Jin, H.-E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef]
- Sophee, S.S.; Prasad, R.G.S.V.; Srinivas, J.V.; Aparna, R.S.L.; Phani, A.R. Antibacterial Activity of TiO2 and ZnO Microparticles Combination on Water Polluting Bacteria. J. Green Sci. Technol. 2013, 1, 20–26. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Zwara, J.; Jaskiewicz, M.; Kowalski, S.; Kamysz, W.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Shape-Depended Biological Properties of Ag3PO4 Microparticles: Evaluation of Antimicrobial Properties and Cytotoxicity in In Vitro Model—Safety Assessment of Potential Clinical Usage. Oxid. Med. Cell. Longev. 2019, 2019, 6740325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Kefallinou, D.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Is There a Threshold in the Antibacterial Action of Superhydrophobic Surfaces? ACS Appl. Mater. Interfaces 2017, 9, 39781–39789. [Google Scholar] [CrossRef]
- Ballo, M.K.S.; Rtimi, S.; Kiwi, J.; Pulgarin, C.; Entenza, J.M.; Bizzini, A. Fungicidal activity of copper-sputtered flexible surfaces under dark and actinic light against azole-resistant Candida albicans and Candida glabrata. J. Photochem. Photobiol. B Biol. 2017, 174, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Akhidime, I.D.; Saubade, F.; Benson, P.S.; Butler, J.A.; Olivier, S.; Kelly, P.; Verran, J.; Whitehead, K.A. The antimicrobial effect of metal substrates on food pathogens. Food Bioprod. Process. 2019, 113, 68–76. [Google Scholar] [CrossRef]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016, 312, 1–7. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of Influenza A Virus on Copper versus Stainless Steel Surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef] [Green Version]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. MBio 2015, 6, e01697-15. [Google Scholar] [CrossRef] [Green Version]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The survival of Escherichia coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rahman Rashid, R.A.; Palanisamy, S. SARS-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Valero, C.; Lagrou, K.; Colombo, A.L.; Goldman, G.H. Potential of Gallium as an Antifungal Agent. Front. Cell. Infect. Microbiol. 2019, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Kao, H.; Chen, C.-C.; Huang, Y.-R.; Chu, Y.-H.; Csík, A.; Ding, S.-J. Metal ion-dependent tailored antibacterial activity and biological properties of polydopamine-coated titanium implants. Surf. Coat. Technol. 2019, 378, 124998. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghandali, N.; Masumeh, S.; Safarkar, R. A review of applications and mechanisms nanoparticles on inhibiting the growth of pathogens. Asian J. Nanosci. Mater. 2021, 4, 67–80. [Google Scholar] [CrossRef]
- Gatadi, S.; Madhavi, Y.V.; Nanduri, S. Nanoparticle drug conjugates treating microbial and viral infections: A review. J. Mol. Struct. 2021, 1228, 129750. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- McShan, D.; Zhang, Y.; Deng, H.; Ray, P.C.; Yu, H. Synergistic Antibacterial Effect of Silver Nanoparticles Combined with Ineffective Antibiotics on Drug Resistant Salmonella typhimurium DT104. J. Environ. Sci. Health Part C 2015, 33, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Golinska, P.; Dahm, H.; Rai, M. Actinobacterial-mediated synthesis of silver nanoparticles and their activity against pathogenic bacteria. IET Nanobiotechnol. 2017, 11, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.K.d.S.; Reis, D.T.; Barbosa, K.M.; Scheidt, G.N.; da Costa, L.S.; Santos, L.S.S. Antibacterial effects and ibuprofen release potential using chitosan microspheres loaded with silver nanoparticles. Carbohydr. Res. 2020, 488, 107891. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.; Pasha, A.; Satish, S. Biogenic nanoparticles bearing antibacterial activity and their synergistic effect with broad spectrum antibiotics: Emerging strategy to combat drug resistant pathogens. Saudi Pharm. J. 2017, 25, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Moteriya, P.; Chanda, S. Biosynthesis of silver nanoparticles formation from Caesalpinia pulcherrima stem metabolites and their broad spectrum biological activities. J. Genet. Eng. Biotechnol. 2018, 16, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J. Appl. Microbiol. 2019, 127, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sportelli, M.C.; Longano, D.; Bonerba, E.; Tantillo, G.; Torsi, L.; Sabbatini, L.; Cioffi, N.; Ditaranto, N. Electrochemical preparation of synergistic nanoantimicrobials. Molecules 2020, 25, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandava, K.; Kadimcharla, K.; Keesara, N.R.; Fatima, S.N.; Bommena, P.; Batchu, U.R. Green Synthesis of Stable Copper Nanoparticles and Synergistic Activity with Antibiotics. Indian J. Pharm. Sci. 2017, 79, 695–700. [Google Scholar] [CrossRef]
- Nejabatdoust, A.; Salehzadeh, A.; Zamani, H.; Moradi-Shoeili, Z. Synthesis, Characterization and Functionalization of ZnO Nanoparticles by Glutamic Acid (Glu) and Conjugation of ZnO@Glu by Thiosemicarbazide and Its Synergistic Activity with Ciprofloxacin Against Multi-drug Resistant Staphylococcus aureus. J. Clust. Sci. 2019, 30, 329–336. [Google Scholar] [CrossRef]
- Ghasemi, N.; Seyfi, J.; Asadollahzadeh, M.J. Imparting superhydrophobic and antibacterial properties onto the cotton fabrics: Synergistic effect of zinc oxide nanoparticles and octadecanethiol. Cellulose 2018, 25, 4211–4222. [Google Scholar] [CrossRef]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanopart. Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Huang, W.; Wang, C.; Duan, H.M.; Bi, Y.L.; Wu, D.; Du, J.L.; Yu, H. Synergistic antifungal effect of biosynthesized silver nanoparticles combined with fungicides. Int. J. Agric. Biol. 2018, 20, 1225–1229. [Google Scholar] [CrossRef]
- Ngoc, U.T.P.; Nguyen, D.H. Synergistic antifungal effect of fungicide and chitosan-silver nanoparticles on Neoscytalidium dimidiatum. Green Process. Synth. 2018, 7, 132–138. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Hua, L.; Wang, H.; Xia, H.; Zhu, B. Silver Nanoparticle Based Codelivery of Oseltamivir to Inhibit the Activity of the H1N1 Influenza Virus through ROS-Mediated Signaling Pathways. ACS Appl. Mater. Interfaces 2016, 8, 24385–24393. [Google Scholar] [CrossRef] [PubMed]
- Alghrair, Z.K.; Fernig, D.G.; Ebrahimi, B. Enhanced inhibition of influenza virus infection by peptide-noble-metal nanoparticle conjugates. Beilstein J. Nanotechnol. 2019, 10, 1038–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Method | Graphical Representation | Short Description | Reference | |
|---|---|---|---|---|
| Top-Down synthesis | Mechanical Milling |  | This approach includes the disintegration of particle aggregates, particle shape, and particle surface. | [20,21,22,23] |
| Chemical Etching |  | Uses a strong acid or a corrosive liquid to cut a metal surface and create a design in the metal. | [24,25] | |
| Sputtering |  | There is a particular sputtering technique called magnetron sputtering, which consists of the delivery of a high voltage across a low-pressure gas (normally argon) to create a plasma of high energy composed of electrons and gas ions, which will strike a target containing the desired coating material. | [26,27] | |
| Laser Ablation |  | Is based on the production of micropatterns through the ablation (removal) of fractions of a substrate through the action of a focused pulsed laser beam. | [20,28] | |
| Electro Explosion |  | Single-step process in which a delicate wire of a conductive metal is exploded by an electric discharge that is caused by a high-power DC source. This electronic discharge creates a massive temperature that vaporizes the thin wire, turning it into gas atoms, which in their turn are chilled and, finally, the NPs are synthesized. | [29,30] | |
| Bottom-up synthesis | Chemical Deposition |  | In particular, electrochemical deposition or electrochemical precipitation involves the passage of an electric current between an anode (sacrificial) and a cathode localized in an electrolyte. The anode is oxidized into metal ions and these are then reduced to metal by the cathode with the help of stabilizers. | [31,32] |
| Spinning |  | This method involves the use of a spinning disc reactor. The disc spins at different speeds and the spinning causes the fusion and precipitation of atoms, which are then collected. | [5,33] | |
| Sol–Gel |  | A wet-chemical process, with sol being a colloidal solution of solids suspended in a liquid phase that serves as a metal precursor and is then dispersed into the gel, a host liquid leading to the formation of a solid macromolecule submerged in the solvent. After, there is a separate phase where the gel is dried and dehydrated to recover the NPs. | [20,24,33,34,35] | |
| Biosynthesis |  | Metalsynthesis is bio-mediated by microbes or through biosynthesis. Biological synthesis of nanomaterials is the best alternative being cost-effective, environmentally friendly, advantageous and does not comprise any input of toxic chemicals. Between the candidates to biosynthesize M-NPs are bacteria, fungi and yeast; algae; plants, flowers and fruits; and viruses. | [18,19,23,36,37,38] |
| NPs | Microbes | Synthesis Method | Applications | Main Results | Reference |
|---|---|---|---|---|---|
| Zn | Gram-positive bacteria: Streptococcus pneumonia, S. aureus, and Bacillus subtilis Gram-negative bacteria: Salmonella typhimurium, Pseudomonas aeruginosa, and E. coli O157:H7 Fungi and Yeast: Candida albicans, Candida glabrata, Candida guilliermondii, and Candida krusei | Green synthesis by Ziziphora clinopodioides extract | Cutaneous wound healing; Medical, biomedical and pharmaceutical research | Antibacterial, antifungal and antioxidant effects, without any cytotoxicity. Wound healing with increased levels of wound contracture, vessel, hydroxyl proline, hexosamine, hexuronic acid and fibrocytes/fibroblast rate. Growth inhibition concentrations were 1 mg/mL for C. guilliermondii and C. krusei, 2 mg/mL for P. aeruginosa, S. aureus, S. pneumonia, B. subtilis, C. albicans and C. glabrata, and 4 mg/mL for E. coli and S. typhimurium. | [54] |
| Gram-positive bacteria: S.aureus, Mycobacterium tuberculosis | Extracellular by Pseudomonas hibiscicola | Drug-resistant strains hospital-acquired infections, treatment and prevention therapy. | Antibacterial potential. Synergism with gentamicin against MRSA. The minimum inhibitory concentrations (MIC) were 2.5 mg/mL for S. aureus and 1.25 mg/mL for M. tuberculosis. | [55] | |
| Virus: Hepatitis A | Hesperidin mediated synthesis | Drug development | Hesperidin-mediated ZnO NPs exhibit better antiviral activity than hesperidin alone. Hesperidin and ZnO NPs showed antiviral activity against Hepatitis A virus (HAV) with EC50s equal to 72.4 and 176.3 μg/mL. | [56] | |
| ZnO | Fungi and Yeast: C. Albicans | Green synthesis by Prosopis farcta aqueous extract | Industrial and medicinal | Antifungal effects and reduction of cell viability in cancer cells by increasing the ZnO-NPs concentration. The antifungal activity of ZnO-NPs against C. Albicans has shown that the MIC and minimum fungicidal concentration (MFC) were 128 and 256 μg/mL, respectively. | [57] |
| Virus: H1N1 Influenza | - | Biomedical | Antiviral activity and reduction of cell cytotoxicity in MDCK-SIAT1 cells with a maximum noncytotoxic concentration of 75 μg/mL. | [58] | |
| Fe | Gram-positive bacteria: S. aureus Gram-negative bacteria: P. aeruginosa, and E. coli | Ferric iron reduction | Biomedical | Strong antibacterial effect considering the MIC values of 1.96, 31.25, and 15.75 μg/mL, and MBC values of 1.96, 31.25, and 31.25 μg/mL for E. coli, P. aeruginosa and S. aureus, respectively. | [59] |
| Vírus: Chikungunya virus (CHIKV) | Green synthesis from impregnation into raw Citrus limetta peels | Therapeutic | Antiviral activities of Fe-NPs at concentrations of 0.05 mg/mL, 0.1 mg/mL, and 0.2 mg/mL and an IC50 value of 15.52 µg/mL. | [60] | |
| Fungi: Fusarium oxysporum, Fusarium tricinctum, Fusarium maniliforme, Rhizoctonia solani, and Phythium sp. | Phyco-synthesis with aqueous extract of the green microalga Chlorella K01 | Biomedical | Iron oxide nanoparticles at 1 mg/L inhibited the radial growth of all fungal pathogens tested. | [61] | |
| Fe3O4 | Fungi: Trichothecium roseum, Cladosporium herbarum, Penicillium chrysogenum, Alternaria alternata and Aspergillus niger | Green synthesis | Biomedical | Antibacterial activity with MIC values of 0.063 mg/mL for Trichothecium roseum and Cladosporium herbarum, 0.032 mg/mL for Alternaria alternata and 0.016 mg/mL for Penicillium chrysogenum. and A.niger. | [62] |
| Vírus: H1N1 influenza | Chemical | Biomedical | A major decrease in viral RNA concentration with the administration of 7.5 pg/mL of iron oxide NPs. | [39] | |
| Ti | Gram-positive bacteria: S. pneumonia, S. aureus, and B. subtilis Gram-negative bacteria: S. typhimurium, P. aeruginosa, and E. coli Fungi and Yeast: C. albicans, C. glabrata, C. guilliermondii, and C. krusei | Green synthesis by Ziziphora clinopodioides extract | Cutaneous wound healing; medical, biomedical and pharmaceutical research | Higher antibacterial and antifungal effects than all standard antibiotics and antioxidant effects. The MIC values were 4 mg/mL for S. typhimurium, E. coli, P. aeruginosa, S. aureus and C. albicans and 2 mg/mL for S. pneumonia, B. subtilis, C. glabrata, C. guilliermondii, and C. krusei. The MBC/MFC the values were 8 mg/mL for S. typhimurium and E. coli, 4 mg/mL for P. aeruginosa, S. aureus, S. pneumonia, C. albicans, C. glabrata and C. guilliermondii and 2 mg/mL for B. subtilis and C. krusei. | [63] |
| TiO2 | Gram-negative bacteria: P. aeruginosa | Green synthesis with the extract of Trichoderma citrinoviridae as a reducing agent | Food, health, and medicine | Inhibit the growth of extremely drug-resistant bacteria at 100 μg/mL and also showed antioxidant potential at this concentration. | [64] |
| MgO | Gram-negative bacteria: E. coli and P. aeruginosa Gram-positive bacteria: Staphylococcus epidermis, S. aureus, Methicillin-resistant S. aureus (MRSA) Fungi and Yeast: C. albicans, C. glabrata | - | Engineering infection-free medical devices and implants | Bactericidal/fungicidal effects. MIC values of 0.5 mg/mL for S. epidermis, 0.7 mg/mL for S. aureus, 1.0 mg/mL for E. coli, P. aeruginosa, MRSA and C. glabrata, and 1.2 mg/mL for C. albicans. Moreover, the minimum lethal concentration (MLC) values were 0.7 mg/mL for S. epidermis and S. aureus, 1.0 mg/mL for E. coli and C. glabrata, 1.2 mg/mL for P. aeruginosa and C. albicans, and 1.4 mg/mL for MRSA. | [44] |
| Gram-positive bacteria: Streptococcus mutans and Streptococcus sobrinus | - | Biomedical and dental | Antibacterial activity and antibiofilm properties. The MIC and MBC values were determined at 500 μg/mL and 1000 μg/mL, respectively, for both S. mutans and S. sobrinus. | [65] | |
| Gram-negative bacteria: E. coli, P.aeruginosa and Aeromonas baumannii Gram-positive bacteria: S. pneumoniae and MRSA Fungi and Yeast: Fusarium solani, A. niger and Aspergillus fumigatus | Green synthesis with marine brown algae Sargassum wighitii as the reducing and capping agent | Biological | Potent antimicrobial activities against both human pathogenic bacterial and fungal strains. MIC for MRSA and P. aeruoginosa was 256 μg/mL and the MBC was observed at 256 and 1024 μg/mL of MgONPs for each bacteria. Antifungal experiments (using 10–30 μg/mL of MgONPs) showed potent antifungal activity when compared to a positive control (Fluconozole). Also presented cytotoxic activity against lung cancer cell lines in a dose-dependent manner. | [66] | |
| Virus: Foot-and-mouth disease | - | Therapeutic antiviral agent in foot-and-mouth disease | Virucidal and antiviral activities in the early steps of the replication cycle before virus entry into the cell. It was observed that concentrations higher than 50 μg/mL inactivate the viruses. | [67] | |
| Cu | Gram-negative bacteria: E. coli and P.aeruginosa Gram-positive bacteria: S. aureus and B. subtilis Fungi and Yeast: C. albicans | Biosynthesis using aqueous Tilia extract | Promising in electronic devices: Human cancer therapy | Cytotoxicity against human colon cancer, human hepatic cancer and human breast cancer cells. Relatively high activity against pathogenic bacteria with concentrations ranging from 25 to 200 μg/mL. | [68] |
| Gram-negative bacteria: E. coli, Vibrio harveyi and Vibrio parahemolyticus Gram-positive bacteria: B. subtilis and S. aureus Fungi and Yeast: R. solani and Sclerotium oryzae | Green synthesis using Manilkara zapota leaf extract | Antiproliferative, antimicrobial and photocatalytic agent | Antiproliferative effect in breast cancer cell line. Fungicidal activity for R. solani and S. oryzae at concentrations of 50 μg/mL, 100 μg/mL and 200 μg/mL. As for antibacterial activity B. subtilis, V. parahaemolyticus, V. harveyi, S. aureus and E. coli presented inhibition at 5 μg/mL concentration of Cu-NPs. | [69] | |
| CuO | Gram-positive bacteria: S. mutans, Lactobacillus casei, and Lactobacillus acidophilus Fungi and Yeast: C. albicans, C. krusei, and C. glabrata | - | Dental | High antimicrobial effect against dental caries bacterial agents with MIC50 values of CuO-NPs determined at the range of 1–10 μg/mL for S. mutans, <1 μg/mL for L. acidophilus, 10 μg/mL for L. casei and 1000 μg/mL for C. albicans, C. krusei, and C. glabrata. | [70] |
| Virus: herpes simplex type 1 | - | Treatment of oro-labial or genital herpetic lesions | Significant antiviral potency against HSV-1, with the production of ROS through free Cu ions released from the NPs, where the optimal concentration for the antiviral effect was found to be 100 μg/mL. | [71] | |
| Cu2O | Gram-negative bacteria: P. aeruginosa Gram-positive bacteria: B. subtilis | Fabricated in reverse micellar templates by using lipopeptidal biosurfactant as a stabilizing agent | Biocompatible bactericidal and therapeutic | Potent antimicrobial activity through significant ROS generation. The MIC value was found to be 62.5 μg/mL for B. subtilis and P. aeruginosa microorganisms. | [72] |
| Au | Gram-negative bacteria: Klebsiella pneumoniae, S. typhimurium, E. coli and P. aeruginosa Gram-positive bacteria: Bacillus cereus, B. subtilis, S. aureus and Corallium rubrum Fungi and Yeast: C. albicans, C. glabrata and Cryptococcus neoformans | Green synthesis using seed extract of mango (Mangifera indica) | Therapeutic agents in the biomedical field | Moderate antibacterial, cytotoxic and antioxidant activity. In addition, it exhibited potential cytotoxicity on cancer cell lines. The inhibition of bacteria and fungi corresponded to concentrations of 50 mg/mL of Au-NPs. | [73] |
| Gram-negative bacteria: E. coli and P. aeruginosa Gram-positive bacteria: B. subtilis and Streptococcus Fungi and Yeast: Aspergillus and Penicillium | Microwave-assisted method using the leaf extract of Synedrella nodiflora as reducing and stabilizing agent | Field of biomedicine and catalysis | Effective antimicrobial activity, significant antioxidant properties and potent catalytic activity. Regarding the antioxidant potential, Au-NPs presented an IC50 of 55.40 μg/mL. | [74] | |
| Virus: Influenza A | Porous Au-NPs were prepared following the surfactant-free emulsion method | M-NPs-based therapy to treat viral infection; Multiplatform for detection of the virus. | Inhibited viral membrane fusion by blocking the viral entry process through conformational deformation of hemagglutinin. The cell viability decreased to less than 60% after 10 min of exposure to 0.2 mg/mL of Au-NPs. | [75] | |
| Virus: Herpes Simplex | Gallic acid-induced rapid reduction reaction. | Virus chemotherapy. | Prevented viral attachment and penetration. The antiviral efficiency had a EC50 value of approximately 32.3 and 38.6 μM for HSV-1 and HSV-2, respectively. | [76] | |
| Ag | Gram-positive bacteria: S.aureus, P. aeruginosa | Extracellular by Pseudomonas hibiscicola | Drug-resistant strains hospital-acquired infections, treatment and prevention therapy. | Profound synergistic antimicrobial activity against drug-resistant strains of MRSA, extended-spectrum ß lactamases producer (ESBL), vancomycin-resistant Enterococci (VRE), and multi-drug resistant (MDR) P. aeruginosa with MIC values of 2.5 mg/mL. | [55] |
| Gram-negative bacteria: E. coli and P. aeruginosa Gram-positive bacteria: B. subtilis and Streptococcus sp. Fungi and Yeast: Aspergillus sp. and Penicillium sp. | Microwave-assisted method using Leaf extract of Synedrella nodiflora as reducing and stabilizing agent | Field of biomedicine and catalysis | Effective antimicrobial activities and significant antioxidant properties. Regarding the antioxidant potential, Ag-NPs presented an IC50 of 54.30 μg/mL | [74] | |
| Gram-negative bacteria: E. coli O157:H7 Gram-positive bacteria: Listeria monocytogenes | Green synthesis with a lyophilized extract from grape and orange wastes | Biomedical | Growth inhibition of bacteria in a dose-dependent manner, with the concentration for inhibition ranging from 20 to 100 μg/mL. | [77] | |
| Gram-negative bacteria: E. coli, P. aeruginosa and Salmonella typhi Gram-positive bacteria: S. aureus and Enterococcus faecalis Fungi and Yeast: A. niger, C. albicans, Penicillium notatum, Trichoderma viridiae and Mucor sp. | Green synthesis using the leaf extracts of the medicinal plant Tropaeolum majus | Therapeutic drug for microbial infectious disease and other health associated disorders | Antibacterial, antifungal, antioxidant and anticancer properties. MIC values were 3 μg/mL for S. typhi, S. aureus, E. faecalis and C. albicans; 4 μg/mL for E. coli and A. niger; 6 μg/mL for P. aeruginosa and 8 μg/mL for P. notatum, T. viridiae and Mucor sp. | [78] | |
| Fungi: Verticillium dahliae | Green synthesis of Ag-NPs using Melia azedarach leaf extract | Horticultural applications | Antifungal activity where an application of 60 ppm of Ag-NPs inhibited mycelial growth with significant effects in vivo. | [79] | |
| Virus: Herpes Simplex types 1 and 2 | Modification with tannic acid | Vaginal treatment of genital infection | Affected viral attachment, blocked penetration and cell-to-cell transmission with an administration of 5 ppm of Ag-NPs modified with Tannic Acid. | [80] | |
| Virus: HAV-10, Herpes Simplex-1 and Coxackie B4 (CoxB4) | Green synthesis by aqueous and hexane extracts of Lampranthus coccineus and Malephora lutea | Therapeutic and biomedical | Antiviral activity by interaction with herpes simplex thymidine kinase, HAV 3c proteinase and Coxsackievirus B4 3c protease. The IC50 of HAV-1, HSV-1 and CoxB4 viruses was 11.71, 36.36 and 12.74 μg/mL, respectively, with Lampranthus coccineus hexane nano extract, and 31.38 and 29.04 μg/mL only for HAV-10 and CoxB4 with Malephora lutea hexane nano extract. | [81] | |
| Virus: Severe acute respiratory syndrome coronavirus 2 | - | Therapeutic | Potent inhibition of viral entry step via disrupting viral integrity with the administration of concentrations ranging from 1–10 ppm of Ag-NPs. | [82] | |
| Virus: Respiratory syncytial virus (RSV) | - | Therapeutic | Reduced viral replication and production of pro-inflammatory cytokines in epithelial cell lines and mouse lungs. A reduction of RSV replication was observed in Hep-2 and A549 epithelial cell lines, with an effective dose of 50 μg/mL Ag-NPs. Also, mouse lung tissue incubated with 4 mg/kg Ag-NPs presented a significant reduction in RSV copy numbers. | [83] | |
| Virus: Human immunodeficiency virus | - | Therapeutic | Exertion of anti-viral activity at an early stage of viral replication and inhibitor of viral entry. The IC50 was calculated at approximately 0.44 mg/mL of Ag-NPs and the CC50 (cytotoxic concentration) in HeLa cells was determined to be approximately 3.9 mg/mL. | [84] | |
| Virus: Human Papilloma virus | Green synthesis using Saccharina japonica extract | Prevention and treatment of cervical tumors | Cytotoxic effect in the human cervical carcinoma cells, where concentrations between 0.16 and 0.32 mg/mL of Ag-NPs could inhibit HeLa cells growth. | [85] |
| Surface | Coating Method | Metals | Strains | Applications | Main Results | Reference |
|---|---|---|---|---|---|---|
| Healthcare | ||||||
| “Hybrid” nanostructured superhydrophobic PMMA surfaces | Sputtering | Ag, Cu | Synechococcus sp. PCC7942 (cyanobacteria) | Hospital, domestic and public surfaces | Metal-sputtered superhydrophobic surfaces able to promote bacterial repulsion and killing efficacy (due to the Ag and Cu ions). | [4,95] |
| Polyester surfaces (PES) | Sputtering | Cu | C. albicans and C. glabrata | Hospital surfaces | Cu-PES displayed fungicidal activity against C. albicans and C. glabrata within 60 min. | [96] |
| Polydopamine-coated Ti implants | Spin/spray coating | Ag+, Cu2+, Sr2+, Zn2+ | E. coli and S. aureus | Dental and orthopaedic prostheses for implants | All ion coatings showed antibacterial activity, reducing the viability of the tested species by over 85% after 3 h of contact. | [105] |
| Laser textured Cu surfaces (LT-Cu) | - (no coating) | Cu | MRSA, P. aeruginosa, S. aureus and E. coli | Biomedical surfaces (e.g., hospital handrails and doorknobs) | LT-Cu eradicated P. aeruginosa and MRSA, E. coli and S. aureus in 40, 90, 60 and 120 min, respectively. | [1] |
| Cu and stainless steel surfaces | - (no coating) | Cu | Influenza A | Surfaces for schools and health care units | Cu inactivated 75% of Influenza A just 1 h, whereas, after 6 h, Cu presented >99.9% viral inactivation. | [99] |
| Food industry and Other applications | ||||||
| Silicon substrates | Sputtering | Ag, Ti, Cu, Fe, Mo, Zn | L. monocytogenes, E. coli and S. aureus | Food industries | Cu killed 99% of all the three strains of bacteria, followed by Ag which killed 36, 99 and 34% of S. aureus, E. coli and L. monocytogenes, respectively. Zn also significantly decreased cell viability of the three strains, Mo and Fe were only effective in killing S. aureus and L. monocytogenes, whilst Ti was only able to kill S. aureus. | [97] |
| Wide range of Cu-containing alloys (Cu, brasses, bronzes, Cu Nis and Cu-Ni-Zn alloys) | - (no coating) | Cu, Zn, Sn, Ni, Al, Mn, Fe, Cr, P, Si, Ti, Mg (in the alloys) | E. coli O157:H7 | Food industries and domestic work surfaces | 19 out of 21 tested alloys eradicated E. coli O157 between 1 to 6 h of contact. Plus, a correlation between Cu content and decreased E. coli O157 survival time was found in the bronzes. | [101] |
| Wide range of Cu-containing alloys (Cus, brasses and other alloys) | - (no coating) | Cu, Zn, Ni, Sn, Fe, Cr, Mn (in the alloys) | HuCoV-229E | Public surfaces | Cu, brasses with Cu >70% and Cu-Ni alloys with Cu >90% had the best virucidal activity, eradicating HuCoV-229E in 20–60 min. Cu ions and ROS formation were responsible for inactivating the virus. | [100] |
| Stainless steel touch surfaces | Cold-spray coating | Cu | SARS-CoV-2 (COVID-19) | Touch surfaces (e.g., stainless-steel door push plates) | Cu inactivated 96% of the virus in the first 2 h of contact and nearly eradicated it after 5 h presenting an inactivation of 99.2%. | [102] |
| In vitro test (microplates) | - (no coating) | Ga3+ | Aspergillus spp. and Candida spp. | Antifungal therapy | Ga displayed potent antifungal potential. | [103] |
| NPs | Type of Drug | Strains | Mechanism of interaction | Main Results | Reference |
|---|---|---|---|---|---|
| Antibacterial | |||||
| Ag | tetracycline, neomycin | S. typhimurium DT104. | The complex formed between drugs and Nps might be produced due to interactions established between the positively charged Ag-NPs and the large amount of OH groups found in the composition of both antibiotics, which provides them with a negative charge. Additionally, Tetracycline and Neomycin can bind to the bacteria’s membrane proteins. | Tetracycline alone in concentration ranges of 0.01–1.25 μg/mL does not inhibit bacterial growth, neither neomycin in ranges of 0–9.6 μg/mL. Ag-NPs alone at 5 μg/mL can cause up to 30% of inhibition after 2 h of exposure. Tetracycline combined with Ag-NPs inhibit bacterial growth by 85% with an IC50 of 0.07 μg/mL after 2 h of exposure. Neomycin combined with Ag-NPs presents an IC50 of 0.43 μg/mL after 2 h of exposure. | [112] |
| streptomycin, amikacin, kanamycin, vancomycin, tetracycline, ampicillin, cefepime, amoxicillin, cefetaxime | S. epidermidis, Serratia marcescens, E. coli, S. typhimurium, K. pneumonia, B. cereus, S. aureus and B. subtilis. | Antibiotics and NPs were used individually and not complexed. | The studied bacteria were found to be inhibited in the presence of the AgNPs and antibiotics combination, which otherwise showed a resistant pattern in the presence of antibiotics (vancomycin, cefetaxime, ampicillin, kanamycin, amikacin, cefepime) alone. | [116] | |
| bacitracin, kanamycin, gentamicin, streptomycin, erythromycin, chloramphenicol (Ch) | B. subtilis, E. coli, S.aureus and K. pneumoniae. | Ag-NPs and antibiotic conjugates can be obtained through electrostatic interactions. It is also possible to have hydrophobic interactions as well as covalent bonds between the NPs and sulfhydryl groups (-SH) present on the antibiotics. | For all the bacteria strains, an overall percentage of synergistic bacterial effect between Ag-NPs and antibiotics was observed with 16, 11.5, 10, 87, 9.4 and 9.7% for kanamycin, gentamicin, streptomycin, bacitracin, ch and erythromycin, respectively. | [117] | |
| chloramphenicol | B. cereus, B. subtilis, S. aureus, C. rubrum, E. coli, P. areuginosa, S.a typhimurium and K. pneumoniae. | The bonding reaction between the antibiotics and AgNPs could occur due to the chelation process. | The MIC values ranged from 0.312–2.5 and 1.25–2.5 mg/mL and the MBC values ranged from 2.5–>10 and 5–10 mg/mL for Ag-NPs and Ch, respectively. However, when combined, the MIC and MBC values decreased for 0.078–0.625 (Ag) and 1.25–10 mg/mL (Ch), respectively. | [118] | |
| Au | cefotaxime and ciprofloxacin | S. typhimurium, S. typhi and Salmonella enteritidis. | Antibiotics and Au-NPs were not complexed. However, the mutual delivery of both species enabled a combined effect of ROS accumulation from the antibiotics effect and membrane disruption, inducing apoptosis due to Au-NPs presence. | S. typhimurium: the MIC value for Au-NPs, cefotaxime and ciprofloxacin alone was 2.5 μg/mL for all. When combined the MIC values were 0.65 μg/mL for Au-NPs and cefotaxime and 0.32 μg/mL for Au-NPs and ciprofloxacin. S. typhi: the MIC values were 5 μg/mL for Au-NPs and cefotaxime alone and 2.5 μg/mL for ciproflaxin alone. When combined the MIC values were 0.65 μg/mL for Au-NPs and cefotaxime and 1.3 μg/mL for Au-NPs and ciprofloxacin. | [119] |
| Cu | benzalkonium chloride (BAC) | E. coli ATCC 25922, S. aureus MRSA 33591 and S. aureus MRSA 25923. | Cu-NPs were prepared by electrochemical synthesis and stabilized by sacrificial anode electrolysis method with benzalkonium chloride, forming core-shell Cu-NPs with BAC as a capping agent. | The obtained results showed an uncountable number of colony-forming units (CFU) for Cu2+ salt (CuCl2), Cu-NPs stabilized with butyl-ammonium perchlorate and tetra-butyl-ammonium perchlorateand; 230 CFUs for BAC alone and 0 CFUs for Cu-NPs combined with BAC. Also, the MIC values for Cu-NPs and BAC combinations are 12.5, <1 and 3.125 μg/mL for E. coli ATCC 25922, S. aureus MRSA 33591 and S. aureus MRSA 25923, respectively. | [120] |
| ampicillin, amoxicillin, gentamicin and ciprofloxacilin | E. coli, S. typhi, Micrococcus luteus and S. mutans | The metallic Cu is known to react with active groups, like amido and hydroxyl, which are present in antibiotic molecules, leading to a synergism between Cu-NPs and antibiotics. | The synergism between Cu-NPs and antibiotics was proved by an increase in the inhibition zone, when both species are combined, presenting 29.06, 7.6, 7.35 and 25.07% of the synergistic activity with ampicilin, amoxicillin, gentamicin and ciprofloxacin, respectively. | [121] | |
| ZnO | glutamic acid, thiosemicarbazide and ciprofloxacin | S. aureus isolates and standard ATCC 25923. | ZnO-NPs were functionalized with glutamic acid and conjugated with thiosemicarbazide, which can react as a chelating ligand. In this case, ZnO-NPs and ciprofloxacin did not form a complex, but instead, functionalized ZnO-NPs damaged the bacterial membrane and removed essential metal ions present on the cell surface by chelation, disrupting the membrane permeability, and allowing ciprofloxacin to enter the bacterial cell. | When administrated alone, ciprofloxacin and ZnO-NPs presented an average inhibition zone significantly lower than when administrated together. | [122] |
| octadecanethiol (ODT) | S. aureus and E. coli. | ZnO-NPs and ODT were not complexed. Possibly, ODT molecules facilitated the dispersion of ZnO-NPs at the fabric’s surface, preventing the formation of agglomerations. | S. aureus: For the ODT and ZnO-NPs alone, the CFU/m2 count was 312000 and 4440, respectively. Whereas for the combination of ODT plus ZnO-NPs, the CFU/m2 count was 600. E. coli: For the ODT and ZnO-NPs alone, the CFU/m2 count was 49800 and 1800, respectively. Whereas for the combination of ODT plus ZnO-NPs, the CFU/m2 count was 48. The combination of ZnO-NPs and the surfactant ODT significantly diminished the bacterial adhesion. | [123] | |
| MgO | nisin | E. coli and Salmonella stanley. | The synergistic effect of MgO-NPs and nisin is not clear. However, nisin treatment might have been responsible for the rupture of large pores in the bacterial cell membrane, allowing the entering of MgO-NPs into the cell, which can produce ROS and damage the bacterial DNA. | E. coli: the logarithmic CFU/mL levels were approximately 3 and 4 when 25 mg/mL of nisin and 4 mg/mL of MgO-NPs, respectively, were administrated alone. Whereas, when they were administrated together, the logarithmic CFU/mL level came down to approximately 1. S.stanley: the logarithmic CFU/mL levels were approximately 3 and 4 when 25 mg/mL of nisin and 4 mg/mL of MgO-NPs, respectively, when administrated alone. Whereas, when they were administrated together, the logarithmic CFU/mL level came down to approximately below 3. | [124] |
| Antifungal | |||||
| Ag | Amphotericin B (AmB) | C. albicans, C. glabrata and C. neoformans. | The bonding reaction between the antifungal and AgNPs might be chelation, which altered the membrane permeability and morphology. | When alone, the MIC values were 2.5 and 5 mg/mL for Ag-NPs and AmB, respectively, whereas the MFC values were 10 and >10 mg/mL for Ag-NPs and AmB, respectively. However, when they were combined, the MIC and MFC values decreased to 0.156 and 2.5 mg/mL, respectively. | [118] |
| tebuconazole, propineb, fludioxonil | Bipolaria maydis | Antifungals and NPs were used individually and not complexed. | The synergism between antifungal compounds and Ag-NPs was measured by the percentage of inhibition towards B. maydis ranging from 46–58% for the Ag-NPs alone 48.28, 47.27 and 52.13% for the fungicides Tebuconazole, Propineb, Fludioxonil, respectively, and with values of 73.08, 64.56 and 62.13% for the combination of Ag-NPs with tebuconazole, propineb, fludioxonil, respectively, therefore proving a synergistic interaction between the NPs and the fungicides. | [125] | |
| zineb | Neoscytalidium dimidiatum. | Chitosan was used to functionalized with Ag-NPs and improve the NPs stability, then this compound was combined with zineb and meant to improve fungicidal effect. | To prove the synergistic effects between Ag-NPs and zineb, the inhibition zones were measured and were high when in combination. | [126] | |
| Antiviral | |||||
| Ag | oseltamivir (OTV) | H1N1 influenza virus. | Ag-NPs and OTV were synthesized together, being the OTV on the surface of the Ag-NPs. It is believed that Ag-NPs can facilitate OTV’s entry into the cell to exert its antiviral action by reducing ROS and p53 levels. | For the infection growth in MDCK cells, the virus alone diminished cell viability to below 40%, virus+OTV presented viability below 60% and the virus+Ag-NPs close to 70%. Virus+Ag-NPs+OTV reached almost 100% of cell viability. Similar results were obtained for the mitochondrial membrane potential, proving the synergism between Ag-NPs and the antiviral OTV. | [127] |
| Ag & Au | FluPep | Influenza type-A virus. | Ag-NPs and Au-NPs were used as probes to deliver FluPep onto the cell. The system was based on a shell of NPs around the peptideFluPep. | Ag-NPs and Au-NPs in combination with the peptide provide better antiviral activity than FluPep alone. FluPep alone had IC50 values ranging from 1–5 nM, whilst in combination with Au-NPs and Ag-NPs, the value of IC50 decreased to 0.015 nM, proving a positive synergistic activity between the antiviral peptide and the NPs. | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.; Carreira, T.S.; Alves, N.; Sousa, Â.; Valente, J.F.A. Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms. Int. J. Mol. Sci. 2022, 23, 1165. https://doi.org/10.3390/ijms23031165
Pereira D, Carreira TS, Alves N, Sousa Â, Valente JFA. Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms. International Journal of Molecular Sciences. 2022; 23(3):1165. https://doi.org/10.3390/ijms23031165
Chicago/Turabian StylePereira, Diana, Tiago Soares Carreira, Nuno Alves, Ângela Sousa, and Joana F. A. Valente. 2022. "Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms" International Journal of Molecular Sciences 23, no. 3: 1165. https://doi.org/10.3390/ijms23031165