Increased Expression of TLR4 in Circulating CD4+T Cells in Patients with Allergic Conjunctivitis and In Vitro Attenuation of Th2 Inflammatory Response by Alpha-MSH
Abstract
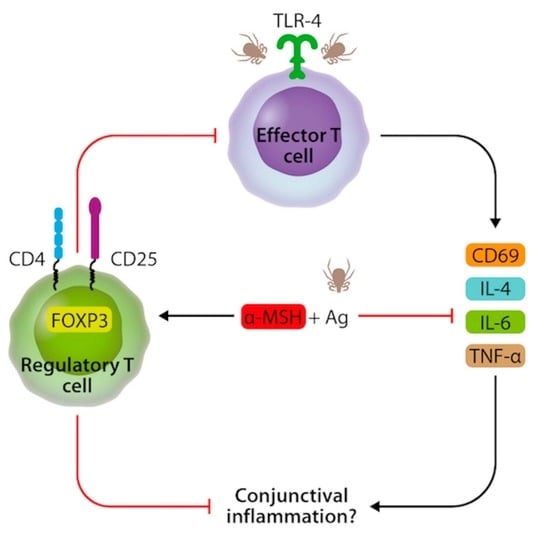
:1. Introduction
2. Results
2.1. Increased Frequency of CD4+TLR4+T Cells in Peripheral Blood Mononuclear Cells in Patients with Perennial Allergic Conjunctivitis
2.2. CD4+TLR4+T Cells Are Induced after Der p Stimulation
2.3. α-MSH Induces Treg Cell Differentiation In Vitro from PBMC after Der p Stimulation
2.4. α-MSH Inhibits the Expression of CD69 in CD4+T Cells and Decreases IL-4 and IL-6 after Der p Stimulation
2.5. Increased Concentration of α-MSH in the Tears of Patients with Allergic Conjunctivitis
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Donors
4.2. Clinical Evaluation
4.3. Monoclonal Antibodies and Reagents
4.4. Immunofluorescence of Cellular Surface Markers
4.5. Immunofluorescence of Intracellular Markers
4.6. Cell Cultures
4.7. Flow Cytometric Analysis
4.8. Quantification of Soluble Cytokines in Culture Supernatants
4.9. Determination of Total IgE (tIgE)
4.10. Tear Samples
4.11. Quantification of α-MSH in Serum and Tears
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miraldi Utz, V.; Kaufman, A.R. Allergic eye disease. Pediatr. Clin. N. Am. 2014, 6, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Rosario, N.; Bielory, L. Epidemiology of allergic conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.J. Trends in prevalence and treatment of ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Ezquerro, M.C.; Guerrero-Guerra, C.; Galán, C.; Serrano-Silva, N.; Guidos-Fogelbach, G.; Jiménez-Martínez, M.C.; Larenas-Linnemann, D.; López-Espinosa, E.D.; Ayala-Balboa, J. Pollen in the atmosphere of Mexico City and its impact on the health of the pediatric population. Atmos. Envrion. 2018, 186, 198–208. [Google Scholar] [CrossRef]
- Reyes, N.; Saban, D. T helper Subsets in Allergic Eye Disease. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Shaker, M.; Salcone, E. An update on ocular allergy. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 505–510. [Google Scholar] [CrossRef]
- Segundo, G.R.; Sopelete, M.C.; Terra, S.A.; Pereira, F.L.; Justino, C.M.; Silva, D.A.; Taketomi, E.A. Diversity of allergen exposure: Implications for the efficacy of environmental control. Braz. J. Otorhinolaryngol. 2009, 75, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A.; Motterle, L.; Bortolotti, M. Allergy and the eye. Clin. Exp. Immunol. 2008, 153 (Suppl. 1), 17–21. [Google Scholar] [CrossRef]
- Magaña, D.; Aguilar, G.; Linares, M.; Ayala-Balboa, J.; Santacruz, C.; Chávez, R.; Estrada-Parra, S.; Garfias, Y.; Lascurain, R.; Jiménez-Martínez, M.C. Intracellular IL-4, IL-5 and IFN-g as the main characteristic of CD4+CD30+T cells after allergen-stimulation, in patients with vernal keratoconjunctivitis. Mol. Vis. 2015, 21, 443–450. [Google Scholar]
- Galicia-Carreón, J.; Santacruz, C.; Ayala-Balboa, J.; Robles-Contreras, A.; Perez-Tapia, S.M.; Garfias, Y.; Hong, E.; Jiménez-Martínez, M.C. An imbalance between frequency of CD4+CD25+FOXP3+ regulatory T cells and CCR4+ and CCR9+ circulating helper T cells is associated with active perennial allergic conjunctivitis. Clin. Dev. Immunol. 2013, 2013, 919742. [Google Scholar] [CrossRef]
- Reithofer, M.; Jahn-Schmid, B. Allergens with Protease Activity from House Dust Mites. Int. J. Mol. Sci. 2017, 18, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakib, F.; Ghaemmaghami, A.M.; Sewell, H.F. The molecular basis of allergenicity. Trends Immunol. 2008, 29, 633–642. [Google Scholar] [CrossRef]
- Redfern, R.L.; McDermott, A.M. Toll-like receptors in ocular surface disease. Exp. Eye Res. 2010, 90, 679–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perros, F.; Lambrecht, B.N.; Hammad, H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir. Res. 2011, 12, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonini, S.; Micera, A.; Iovieno, A.; Lambiase, A.; Bonini, S. Expression of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmology 2005, 112, 1528, discussion 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Miyata, S.; Itoh, Y.; Mizuki, N.; Ohgami, K.; Shiratori, K.; Ilieva, I.B.; Ohno, S.; Taylor, A.W. Anti-inflammatory effects of alpha-melanocyte-stimulating hormone against rat endotoxin-induced uveitis and the time course of inflammatory agents in aqueous humor. Int. Immunopharmacol. 2004, 4, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. The immunomodulating neuropeptide alpha-melanocyte-stimulating hormone (alpha-MSH) suppresses LPS-stimulated TLR4 with IRAK-M in macrophages. J. Neuroimmunol. 2005, 162, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Namba, K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH). Immunol. Cell Biol. 2001, 79, 358–367. [Google Scholar] [CrossRef]
- Trompette, A.; Divanovic, S.; Visintin, A.; Blanchard, C.; Hegde, R.S.; Madan, R.; Thorne, P.S.; Wills-Karp, M.; Gioannini, T.L.; Weiss, J.P.; et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009, 457, 585–588. [Google Scholar] [CrossRef]
- Thomas, W.R.; Hales, B.J.; Smith, W.-A. House dust mite allergens in asthma and allergy. Trends Mol. Med. 2010, 16, 321–328. [Google Scholar] [CrossRef]
- Zanin-Zhorov, A.; Tal-Lapidot, G.; Cahalon, L.; Cohen-Sfady, M.; Pevsner-Fischer, M.; Lider, O.; Cohen, I.R. Cutting edge: T cells respond to lipopolysaccharide innately via TLR4 signaling. J. Immunol. 2007, 179, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Pratheek, B.M.; Meena, V.S.; Nayak, T.K.; Kumar, P.S.; Bandyopadhyay, S.; Maiti, P.K.; Chattopadhyay, S. VIPER regulates naive T cell activation and effector responses: Implication in TLR4 associated acute stage T cell responses. Sci. Rep. 2018, 8, 7118. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.K.; Jeong-Eun, K.; Eui-Cheol, S. IL-17A-producing Foxp3+ regulatory T cells and human diseases. Immune Netw. 2017, 17, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasare, C.; Ruslan, M. Toll pathway-dependent blockade of CD4+ CD25+T cell-mediated suppression by dendritic cells. Science 2003, 299, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.S.; Liang, Y.; Tyznik, A.J.; Self, S.G.; Liggitt, D.; Rudensky, A.Y. Recognition of the peripheral self by naturally arising CD25+CD4+T cell receptors. Immunity 2004, 21, 267–277. [Google Scholar] [CrossRef] [Green Version]
- La Cava, A. Tregs are regulated by cytokines: Implications for autoimmunity. Autoimmun. Rev. 2008, 8, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sancho, D.; Gómez, M.; Sánchez-Madrid, F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005, 26, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Han, D.; Hong, J.; Zhang, H.; Ying, Y.; Tian, Y.; Zhang, L.; Lin, J. SVα-MSH, a novel α-melanocyte stimulating hormone analog, ameliorates autoimmune encephalomyelitis through inhibiting autoreactive CD4+T cells activation. J. Neuroimmunol. 2014, 269, 9–19. [Google Scholar] [CrossRef]
- Raap, U.; Brzoska, T.; Sohl, S.; Päth, G.; Emmel, J.; Herz, U.; Braun, A.; Luger, T.; Renz, H. α-Melanocyte-stimulating hormone inhibits allergic airway inflammation. J. Immunol. 2003, 171, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Liew, L.N.; Kuo, I.C.; Huang, C.H.; Goh, D.L.M.; Chua, K.Y. The modulatory effects of lipopolysaccharide-stimulated B cells on differential T-cell polarization. Immunology 2008, 125, 218–228. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, Y.; Huang, Y.; Liu, H.; Du, J.; Meng, Z.; Dou, Z.; Liu, X.; Wei, R.H.; Zhang, Y.; Zhao, S. α-Melanocyte-stimulating hormone ameliorates ocular surface dysfunctions and lesions in a scopolamine-induced dry eye model via PKA-CREB and MEK-Erk pathways. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiner, S.; Braunstahl, G.J.; Rüdrich, U.; Gehring, M.; Eiz-Vesper, B.; Luger, T.A.; Steelant, B.; Seys, S.F.; Kapp, A.; B€ohm, M.; et al. Regulation of melanocortin 1 receptor in allergic rhinitis in vitro and in vivo. Clin. Exp. Allergy 2016, 46, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Chidi-Egboka, N.C.; Briggs, N.E.; Jalbert, I.; Golebiowski, B. The ocular surface in children: A review of current knowledge and meta-analysis of tear film stability and tear secretion in children. Ocul. Surf. 2019, 17, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Fukuoka, S.; Morishige, N. New Insights into the Lipid Layer of the Tear Film and Meibomian Glands. Eye Contact Lens. 2017, 43, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.H.; Anthonavage, M.; Pappas, A.; Rossetti, D.; Cavender, D.; Seiberg, M.; Eisinger, M. Melanocortin-5 receptor and sebogenesis. Eur. J. Pharmacol. 2011, 660, 202–206. [Google Scholar] [CrossRef]
- House, J.S.; Zhu, S.; Ranjan, R.; Linder, K.; Smart, R.C. C/EBPalpha and C/EBPbeta are required for Sebocyte differentiation and stratified squamous differentiation in adult mouse skin. PLoS ONE 2010, 5, e9837. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.N.; Ryu, J.H.; Joo, J.H.; Choi, Y.H.; Lee, H.J.; Kim, Y.J.; Kim, K.B.; Yoon, J.H. α-Melanocyte-stimulating hormone inhibits tumor necrosis factor α-stimulated MUC5AC expression in human nasal epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011, 44, 716–724. [Google Scholar] [CrossRef]
- Salazar, A.; Casanova-Méndez, I.; Pacheco-Quito, M.; Velázquez-Soto, H.; Ayala-Balboa, J.; Graue-Hernández, E.O.; Serafín-López, J.; Jiménez-Martínez, M.C. Low Expression of IL-10 in Circulating Bregs and Inverted IL-10/TNF-α Ratio in Tears of Patients with Perennial Allergic Conjunctivitis: A Preliminary Study. Int. J. Mol. Sci. 2019, 20, 1035. [Google Scholar] [CrossRef] [Green Version]
- Santacruz, C.; Linares, M.; Garfias, Y.; Loustaunau, L.M.; Pavon, L.; Perez-Tapia, S.M.; Jimenez-Martinez, M.C. Expression of IL-8, IL-6 and IL-1β in Tears as a Main Characteristic of the Immune Response in Human Microbial Keratitis. Int. J. Mol. Sci. 2015, 16, 4850–4864. [Google Scholar] [CrossRef]





| Demographic Characteristics | HD (n = 9) MD (IQR) | PAC (n = 14) MD (IQR) | p Value |
|---|---|---|---|
| Age | 13 (12–14.5) | 11 (10–12) | NS |
| Male | 14 (12–17.5) | 11.2 (9–12.75) | NS |
| Female | 16.5 (13.7–19.2) | 11 (10–12) | NS |
| TBUT (s) | 7.5 (6–10) | 5 (4–6) | * 0.0010 |
| Schirmer Test (mm) | 25.8 (20–35) | 21 (11.25–28) | NS |
| SPT (mm) to Der p | 2.7 (0–7.5) | 7.3 (5–9) | ** 0.0276 |
| tIgE | 49 (19.2–59) | 612.3 (842.8–299.8) | <0.0001 |
| T Cells Subsets % | HD (n = 9) MD (IQR) | PAC (n = 14) MD (IQR) | p Value |
|---|---|---|---|
| CD4+ | 33.45 (26.35–39.2) | 35.65 (27.85–38.8) | NS |
| CD4+TLR4+ | 2.6 (1.3–4.3) | 5.6 (3–39.83) | * 0.0289 |
| CD4+CD25+ | 10.27 (9.31–14.02) | 16.7 (12.95–23.15) | ** 0.0042 |
| CD4+CD25+TLR4+ | 2.4 (1.8–3.4) | 22.65 (3–49) | * 0.0218 |
| CD4+CD25-TLR4+ | 2.12 (0.7–3.4) | 7 (1.8–31.2) | NS |
| CD4+CD25+FOXP3+ | 9.9 (4–10) | 1 (0.39–1.5) | *** 0.0001 |
| T Cells Subsets MFI | HD (n = 9) MD (IQR) | PAC (n = 14) MD (IQR) | p Value |
|---|---|---|---|
| TLR4 | 412 (349–566) | 491 (356–10524.75) | * 0.0167 |
| FOXP3 | 5333 (1804–6435) | 1229 (831–1877) | * 0.0143 |
| T Cells Subsets% | RPMI MD (IQR) | Der p MD (IQR) | LPS MD (IQR) | Der p + LPS MD (IQR) | α-MSH MD (IQR) | Der p + α-MSHMD (IQR) | LPS + α-MSH MD (IQR) | Der p + LPS + α-MSH MD (IQR) | Con A MD (IQR) | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| CD4+CD69+ | 1.35 (0.76–2.4) | 2.9 (2.2–3.8) | 2.6 (1.9–5.6) | 3.8 (1.8–11.3 | 1.68 (0.52–2.9) | 1.6 (0.5–2.3) | 3–045 (1.98–4.68) | 4 (1.8–11.3) | 50.6 (37.8–64.3) | a ** p = 0.0057, b * p = 0.0147, c ** p = 0.0056 |
| CD4+TLR4+ | 1.7 (0.4–2.24) | 5.4 (2.7–6.2) | 4.5 (3.2–9.6) | 7.5 (3.7–9.9) | 2 (1.3–3.4) | 4 (2–5.3) | 1.4 (0.6–3.5) | 6.3 (2–10.7) | 35.7 (12.3–62) | a * p = 0.0108, b ** p = 0.0087, c p = 0.5238 |
| CD4+CD25+ | 5.18 (2.8–5.7) | 10.57 (5.9–14.68) | 9.2 (7.7–12.9) | 8.22 (7.6–14.35) | 5.15 (4–6.2) | 9.18 (7.3–12) | 8 (5.9–10.4) | 14.85 (13.5–16.3) | 53 (27.8–62.5) | a ** p = 0.0059, b *** p = 0.0003, c p = 0.9038 |
| CD4+ CD25+ TLR4+ | 0.05 (0.02–0.35) | 1 (0.15–2.4) | 10.7 (8–13) | 8.2 (7.8–15.3) | 0.06 (0.01–0.51) | 0.54 (0.23–1.5) | 0.14 (0.06–0.6) | 1.4 (1.06–1.8) | 44 (3.3–59) | ap = 0.0303, b * p = 0.0173, c p = 0.668 |
| CD4+CD25-TLR4+ | 2.3 (1.2–5.6) | 5.8 (4.8–12.8) | 4 (2.1–6) | 4.2 (3.1–9.6) | 5.8 (1.8–10.27) | 4.2 (3.1–9.6) | 2.8 (1.7–4.1) | 1.4 (0.82–7.7) | 6.6 (4.2–7.6) | a * p = 0.0325, b p = 0.5281, c p = 0.3333 |
| CD4+CD25+FOXP3+ | 0.32 (0.23–1.32) | 3.3 (2.7–3.9) | 1.1 (1.05–2.6) | 3.75 (1.6–4.3) | 0.5 (0.42–2.09) | 4.6 (3.02–5.4) | 2.08 (0.97–3.5) | 4.8 (2.4–9.4) | 6.7 (3.4–7.5) | a ** p = 0.0013, b p = 0.0958, c * p = 0.0478 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, J.E.; Casanova, I.; Serna-Ojeda, J.C.; Graue-Hernández, E.O.; Quintana, G.; Salazar, A.; Jiménez-Martinez, M.C. Increased Expression of TLR4 in Circulating CD4+T Cells in Patients with Allergic Conjunctivitis and In Vitro Attenuation of Th2 Inflammatory Response by Alpha-MSH. Int. J. Mol. Sci. 2020, 21, 7861. https://doi.org/10.3390/ijms21217861
Nieto JE, Casanova I, Serna-Ojeda JC, Graue-Hernández EO, Quintana G, Salazar A, Jiménez-Martinez MC. Increased Expression of TLR4 in Circulating CD4+T Cells in Patients with Allergic Conjunctivitis and In Vitro Attenuation of Th2 Inflammatory Response by Alpha-MSH. International Journal of Molecular Sciences. 2020; 21(21):7861. https://doi.org/10.3390/ijms21217861
Chicago/Turabian StyleNieto, Jane E., Israel Casanova, Juan Carlos Serna-Ojeda, Enrique O. Graue-Hernández, Guillermo Quintana, Alberto Salazar, and María C. Jiménez-Martinez. 2020. "Increased Expression of TLR4 in Circulating CD4+T Cells in Patients with Allergic Conjunctivitis and In Vitro Attenuation of Th2 Inflammatory Response by Alpha-MSH" International Journal of Molecular Sciences 21, no. 21: 7861. https://doi.org/10.3390/ijms21217861