Analysis of Protein Conformational Strains—A Key for New Diagnostic Methods of Human Diseases
Abstract
:1. Introduction
2. Conformational Diseases
3. Synucleinopathies
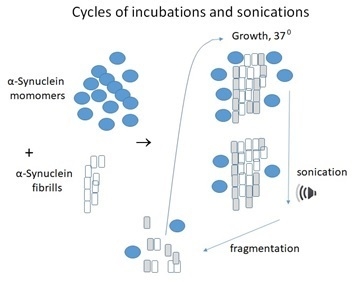
4. Protein Misfolding Cyclic Amplification as an Early Diagnostic Method
5. PD and MSA Can Be Differentiated at an Early Stage
6. Comparison of PMCA with Real-Time Quaking-Induced Conversion (RT-QuIC) Assay
Funding
Acknowledgments
Conflicts of Interest
References
- Kopito, R.R.; Ron, D. Conformational disease. Nat. Cell Biol. 2000, 2, E207–E209. [Google Scholar] [CrossRef] [PubMed]
- Surguchev, A.; Surguchov, A. Conformational diseases: Looking into the eyes. Brain Res. Bull. 2010, 81, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, J.S.; Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Model. Mech. 2014, 7, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1341–1349. [Google Scholar] [CrossRef]
- Koga, S.; Aoki, N.; Uitti, R.J.; Van Gerpen, J.A.; Cheshire, W.P.; Josephs, K.A.; Wszolek, Z.K.; Langston, J.W.; Dickson, D.W. When DLB, PD, and PSP masquerade as MSA: An autopsy study of 134 patients. Neurology 2015, 85, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years On. J. Park. Dis. 2017, 7, S51–S69. [Google Scholar] [CrossRef] [Green Version]
- Courte, J.; Bousset, L.; Von Boxberg, Y.; Villard, C.; Melki, R.; Peyrin, J.-M. The expression level of alpha-synuclein in different neuronal populations is the primary determinant of its prion-like seeding. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Halliday, G.M.; Holton, J.L.; Revesz, T.; Dickson, D.W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011, 122, 187–204. [Google Scholar] [CrossRef]
- Saborio, G.P.; Permanne, B.; Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 2001, 411, 810–813. [Google Scholar] [CrossRef]
- Soto, C.; Saborio, G.P.; Anderes, L. Cyclic amplification of protein misfolding: Application to prion-related disorders and beyond. Trends Neurosci. 2002, 25, 390–394. [Google Scholar] [CrossRef]
- Castilla, J.; Saá, P.; Hetz, C.; Soto, C. In Vitro Generation of Infectious Scrapie Prions. Cell 2005, 121, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, R.; Duran-Aniotz, C.; Diaz-Espinoza, R.; Camacho, M.V.; Soto, C. Protein misfolding cyclic amplification of infectious prions. Nat. Protoc. 2012, 7, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Saa, P.; Castilla, J.; Soto, C. Ultra-efficient Replication of Infectious Prions by Automated Protein Misfolding Cyclic Amplification. J. Boil. Chem. 2006, 281, 35245–35252. [Google Scholar] [CrossRef] [Green Version]
- Bieschke, J.; Weber, P.; Sarafoff, N.; Beekes, M.; Giese, A.; Kretzschmar, H. Autocatalytic self-propagation of misfolded prion protein. Proc. Natl. Acad. Sci. USA 2004, 101, 12207–12211. [Google Scholar]
- Roostaee, A.; Beaudoin, S.; Staskevicius, A.; Roucou, X. Aggregation and neurotoxicity of recombinant α-synuclein aggregates initiated by dimerization. Mol. Neurodegener. 2013, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Herva, M.E.; Zibaee, S.; Fraser, G.; Barker, R.A.; Goedert, M.; Spillantini, M.G. Anti-amyloid compounds inhibit α-synuclein aggregation induced by protein misfolding cyclic amplification (PMCA). J. Boil. Chem. 2014, 289, 11897–11905. [Google Scholar] [CrossRef] [Green Version]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Meng, L.; Zhang, Z. What is strain in neurodegenerative diseases? Cell. Mol. Life Sci. 2019, 77, 665–676. [Google Scholar] [CrossRef]
- Peng, C.; Gathagan, R.; Covell, D.; Medellin, C.; Stieber, A.; Robinson, J.L.; Zhang, B.; Pitkin, R.M.; Olufemi, M.F.; Luk, K.C.; et al. Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 2018, 557, 558–563. [Google Scholar] [CrossRef]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Böckmann, A.; Meier, B.H.; et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, A.; So, R.W.L.; Lau, H.H.C.; Sang, J.C.; Riquelme, A.I.R.; Fleck, S.C.; Stuart, E.; Menon, S.; Visanji, N.P.; Meisl, G.; et al. α-Synuclein strains target distinct brain regions and cell types. Nat. Neurosci. 2019, 23, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Condello, C.; Lemmin, T.; Stöhr, J.; Nick, M.; Wu, Y.; Maxwell, A.M.; Watts, J.C.; Caro, C.D.; Oehler, A.; Keene, C.D.; et al. Structural heterogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E782–E791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, D.; Kaufman, S.K.; Devos, S.L.; Sharma, A.M.; Mirbaha, H.; Li, A.; Barker, S.J.; Foley, A.C.; Thorpe, J.; Serpell, L.C.; et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 2014, 82, 1271–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicot, S.; Verchère, J.; Bélondrade, M.; Mayran, C.; Bétemps, D.; Bougard, D.; Baron, T. Seeded propagation of α-synuclein aggregation in mouse brain using protein misfolding cyclic amplification. FASEB J. 2019, 33, 12073–12086. [Google Scholar] [CrossRef] [Green Version]
- Espay, A.J.; Vizcarra, J.A.; Marsili, L.; Lang, A.E.; Simon, D.K.; Merola, A.; Josephs, K.A.; Fasano, A.; Morgante, F.; Savica, R.; et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology 2019, 92, 329–337. [Google Scholar] [CrossRef]
- Atarashi, R.; Wilham, J.M.; Christensen, L.; Hughson, A.G.; A Moore, R.; Johnson, L.M.; A Onwubiko, H.; A Priola, S.; Caughey, B. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat. Methods 2008, 5, 211–212. [Google Scholar] [CrossRef]
- Davenport, K.A.; Hoover, C.E.; Denkers, N.D.; Mathiason, C.; Hoover, E.A. Modified Protein Misfolding Cyclic Amplification Overcomes Real-Time Quaking-Induced Conversion Assay Inhibitors in Deer Saliva To Detect Chronic Wasting Disease Prions. J. Clin. Microbiol. 2018, 56, e00947-18. [Google Scholar] [CrossRef] [Green Version]
- Hoover, C.E.; Davenport, K.A.; Henderson, D.M.; Zabel, M.D.; Hoover, E.A. Endogenous Brain Lipids Inhibit Prion Amyloid Formation In Vitro. J. Virol. 2017, 91, e02162-16. [Google Scholar] [CrossRef] [Green Version]
- Haley, N. Amplification Techniques for the Detection of Misfolded Prion Proteins in Experimental and Clinical Samples. Curr. Protoc. Mol. Boil. 2020, 130, e118. [Google Scholar] [CrossRef] [Green Version]
- Paciotti, S.; Bellomo, G.; Gatticchi, L.; Parnetti, L. Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of “Protein-Misfolding Cyclic Amplification” and “Real-Time Quaking-Induced Conversion” as Diagnostic Tools. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surguchov, A. Analysis of Protein Conformational Strains—A Key for New Diagnostic Methods of Human Diseases. Int. J. Mol. Sci. 2020, 21, 2801. https://doi.org/10.3390/ijms21082801
Surguchov A. Analysis of Protein Conformational Strains—A Key for New Diagnostic Methods of Human Diseases. International Journal of Molecular Sciences. 2020; 21(8):2801. https://doi.org/10.3390/ijms21082801
Chicago/Turabian StyleSurguchov, Andrei. 2020. "Analysis of Protein Conformational Strains—A Key for New Diagnostic Methods of Human Diseases" International Journal of Molecular Sciences 21, no. 8: 2801. https://doi.org/10.3390/ijms21082801