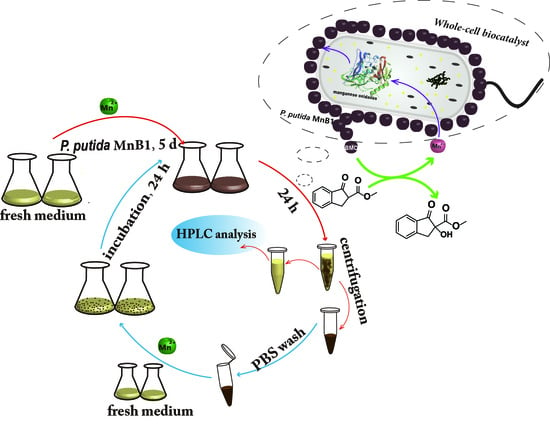
Manganese(II) Oxidizing Bacteria as Whole-Cell Catalyst for β-Keto Ester Oxidation
Abstract
:1. Introduction
2. Results
2.1. Biogenic Manganese Oxides (BMO) Formation
2.2. Reactivity Comparison of the Dry m-MnB1 with Chemical Manganese Oxides (CMO) toward the Oxidation of β-Keto Ester
2.2.1. Solvent Effects on Oxidation Rate
2.2.2. Influence of Dosage of Manganese Oxides on Reaction Rate
2.2.3. Reaction Kinetics
2.3. Effects of β-Keto Ester 1 on Bacteria Growth
2.4. Effects of β-Keto Ester 1 on Manganese Mineralization
2.5. Continuous Live MnB1 Catalyzed Synthesis of α-Hydroxy-β-Keto Ester
3. Discussion
4. Materials and Methods
4.1. Preparation of Freeze-Dried BMO
4.2. Quantification of Freeze-Dried BMO by Inductive Coupled Plasma Optical Emission Spectrometry (ICP-OES)
4.3. Synthesis of α-Hydroxy-β-Keto Ester by the Dry m-MnB1 and CMO
4.3.1. Optimization of the Reaction Medium
4.3.2. Assays of Different Dosage of Manganese Oxides on the Oxidation Reaction
4.3.3. Kinetic Measurements of Dry m-MnB1 and CMO
4.4. Bioassays of Substrate β-Keto Ester Tolerance of Pseudomonas putida MnB1
4.5. Investigation of the Effects of Substrate on Manganese Mineralization
4.6. Continuous Biocatalytic Experiments by Whole Cells Based on BMO
4.7. Methods and Conditions of HPLC Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMO | Biogenic manganese oxide |
| CMO | Chemical manganese oxide |
| MeCN | Acetonitrile |
| CFU | Colony forming unit |
| ICP-OES | Inductive coupled plasma optical emission spectrometry |
| TFA | Trifluoroacetic acid |
References
- Sheldon, R.A.; Brady, D. Broadening the scope of biocatalysis in sustainable organic synthesis. ChemSusChem 2019, 12, 2859–2881. [Google Scholar] [CrossRef] [PubMed]
- Son, E.J.; Lee, Y.W.; Ko, J.W.; Park, C.B. Amorphous Carbon Nitride as a Robust Photocatalyst for Biocatalytic Solar-To-Chemical Conversion. ACS Sustain. Chem. Eng. 2018, 7, 2545–2552. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Du, J.; Liu, K.; Wang, T.; Liu, L. Biocatalytic production of 2, 5-Furandicarboxylic acid: Recent advances and future perspectives. Appl. Microbiol. Biot. 2020, 104, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, B.A.; Hyster, T.K. Emerging strategies for expanding the toolbox of enzymes in biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 45–51. [Google Scholar] [CrossRef]
- Skellam, E. Strategies for engineering natural product biosynthesis in fungi. Trends Biotechnol. 2019, 37, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Woodley, J.M. New frontiers in biocatalysis for sustainable synthesis. Curr. Opin. Green Sustain. Chem. 2020, 21, 22–26. [Google Scholar] [CrossRef]
- Akanuma, S.; Bessho, M.; Kimura, H.; Furukawa, R.; Yokobori, S.-I.; Yamagishi, A. Establishment of mesophilic-Like catalytic properties in a thermophilic enzyme without affecting its thermal stability. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hepworth, L.; France, S.; Hussain, S.; Both, P.; Turner, N.; Flitsch, S. De Novo Enzyme Cascades in Whole Cells for the Synthesis of Chiral Cyclic Amines. ACS Catal. 2017, 4, 2920–2925. [Google Scholar] [CrossRef] [Green Version]
- Duetz, W.A.; Van Beilen, J.B.; Witholt, B. Using proteins in their natural environment: Potential and limitations of microbial whole-Cell hydroxylations in applied biocatalysis. Curr. Opin. Biotechnol. 2001, 12, 419–425. [Google Scholar] [CrossRef]
- Liao, X.; Hou, J.; Wang, Y.; Zhang, H.; Sun, Y.; Li, X.; Tang, S.; Kato, K.; Yamauchi, M.; Jiang, Z. An active, selective, and stable manganese oxide-supported atomic Pd catalyst for aerobic oxidation of 5-hydroxymethylfurfural. Green Chem. 2019, 21. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Renger, G.; Hołyńska, M.; Moghaddam, A.N.; Aro, E.-M.; Carpentier, R.; Nishihara, H.; Eaton-Rye, J.J.; Shen, J.-R.; Allakhverdiev, S.I. Manganese compounds as water-Oxidizing catalysts: From the natural water-Oxidizing complex to nanosized manganese oxide structures. Chem. Rev. 2016, 116, 2886–2936. [Google Scholar] [CrossRef] [PubMed]
- Reidies, A.H. Manganese Compounds; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Yu, Q.; Fu, Y.; Huang, J.; Qin, J.; Zuo, H.; Wu, Y.; Zhong, F. Enantioselective Oxidative Phenol-Indole [3+ 2] Coupling Enabled by Biomimetic Mn (III)/Brønsted Acid Relay Catalysis. ACS Catal. 2019, 9, 7285–7291. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, C.; Chen, J.; Chen, X. Oxidative transformation of 17β-Estradiol by MnO2 in aqueous solution. Arch. Environ. Contam. Toxicol. 2009, 57, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Wu, M.; Zong, W.; Yi, X.; Zhan, J.; Liu, L.; Zhou, H. Coupling the phenolic oxidation capacities of a bacterial consortium and in situ-generated manganese oxides in a moving bed biofilm reactor (MBBR). Water Res. 2019, 166, 115047. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.-H. Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ. Sci. Technol. 2003, 37, 2421–2430. [Google Scholar] [CrossRef]
- Tran, T.N.; Kim, D.-G.; Ko, S.-O. Synergistic effects of Biogenic Manganese Oxide and Mn(II)-Oxidizing bacterium Pseudomonas putida strain MnB1 on the degradation of 17 α-Ethinylestradiol. J. Hazard. Mater. 2018, 344, 350–359. [Google Scholar] [CrossRef]
- Zhou, D.; Kim, D.-G.; Ko, S.-O. Heavy metal adsorption with biogenic manganese oxides generated by Pseudomonas putida strain MnB1. J. Eng. Chem. 2015, 24, 132–139. [Google Scholar] [CrossRef]
- Caspi, R.; Tebo, B.M.; Haygood, M.G. c-Type Cytochromes and Manganese Oxidation in Pseudomonas putida MnB1. Appl. Environ. Microbiol. 1998, 64, 3549–3555. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ruiz, E.B.; Cooper, M.; Fastner, J.; Szewzyk, U. Manganese-Oxidizing bacteria isolated from natural and technical systems remove cylindrospermopsin. Chemosphere 2020, 238, 124625. [Google Scholar] [CrossRef]
- Su, J.; Deng, L.; Huang, L.; Guo, S.; Liu, F.; He, J. Catalytic oxidation of manganese (II) by multicopper oxidase CueO and characterization of the biogenic Mn oxide. Water Res. 2014, 56, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Tsang, Y.F.; Wang, H.; Sun, Y.; Song, Y.; Pan, X.; Luo, S. Effective stabilization of arsenic in contaminated soils with biogenic manganese oxide (BMO) materials. Environ. Pollut. 2019. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, M.; Lanson, B.; Manceau, A.; Toner, B.; Sposito, G. Structural model for the biogenic Mn oxide produced by Pseudomonas putida. Am. Mineral. 2006, 91, 489–502. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. Recent topics in the syntheses of β-keto carboxylic acids and the derivatives. Tetrahedron Lett. 2018, 59, 1295–1300. [Google Scholar] [CrossRef]
- Ren, Q.; Huang, J.; Wang, L.; Li, W.; Liu, H.; Jiang, X.; Wang, J. Highly Efficient Assembly of 3-Hydroxy Oxindole Scaffold via a Catalytic Decarboxylative [1,2]-Addition Strategy. ACS Catal. 2012, 2, 2622–2625. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Tang, X.; Wu, Y.; Yu, Z.; Meng, Q. Visible Light-Induced Salan-Copper (II)-Catalyzed Enantioselective Aerobic α-Hydroxylation of β-Keto Esters. Adv. Syn. Catal. 2019, 361, 1673–1677. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, D.; Lu, Y.; Zeng, X.; Tan, B.; Xu, Z.; Zhong, G. Chiral Brønsted acid-Catalyzed enantioselective α-Hydroxylation of β-Dicarbonyl compounds. J. Am. Chem. Soc. 2009, 131, 4562–4563. [Google Scholar] [CrossRef]
- Naganawa, Y.; Aoyama, T.; Kato, K.; Nishiyama, H. Cu (II)-Catalyzed Enantioselective α-Hydroxylation and α-Chlorination of β-Ketoesters with N, N, O-Tridentate Chiral Phenanthroline Ligand. Chem. Sel. 2016, 1, 1938–1942. [Google Scholar]
- Yang, F.; Zhao, J.; Tang, X.; Zhou, G.; Song, W.; Meng, Q. Enantioselective α-Hydroxylation by Modified Salen-Zirconium (IV)-Catalyzed Oxidation of β-Keto Esters. Organic Lett. 2017, 19, 448–451. [Google Scholar] [CrossRef]
- Yin, X.P.; Zhu, L.; Zhou, J. Metal-Free Azidation of α-Hydroxy Esters and α-Hydroxy Ketones Using Azidotrimethylsilane. Adv. Syn. Catal. 2018, 360, 1116–1122. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Yu, H.; Bi, J.; Zhang, G. Tandem Oxidative α-Hydroxylation/β-Acetalization Reaction of β-Ketoamides and Its Applications. ACS Omega 2017, 2, 7746–7754. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, B.; Mu, H.; Zhang, H.; Song, Y.; Qu, J. Development of tartaric acid derived chiral guanidines and their application to catalytic enantioselective a-hydroxylation of b-dicarbonyl compounds. Org. Lett. 2013, 15, 3106–3109. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.A.; Tebo, B.M. cumA Multicopper Oxidase Genes from Diverse Mn (II)-Oxidizing and Non-Mn (II)-OxidizingPseudomonas Strains. Appl. Environ. Microbiol. 2001, 67, 4272–4278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.K.; Schweisfurth, R. Manganese oxidation by an intracellular protein of a Pseudomonas species. J. Basic Microb. 2007, 19, 107–115. [Google Scholar]
- Soldatova, A.V.; Romano, C.A.; Tao, L.; Stich, T.A.; Casey, W.H.; Britt, R.D.; Tebo, B.M.; Spiro, T.G. Mn (II) oxidation by the multicopper oxidase complex Mnx: A coordinated two-Stage Mn (II)/(III) and Mn (III)/(IV) mechanism. J. Am. Chem. Soc. 2017, 139, 11381–11391. [Google Scholar] [CrossRef]
- Grobler, J.A.; Stillmock, K.; Hu, B.; Witmer, M.; Felock, P.; Espeseth, A.S.; Wolfe, A.; Egbertson, M.; Bourgeois, M.; Melamed, J. Diketo acid inhibitor mechanism and HIV-1 integrase: Implications for metal binding in the active site of phosphotransferase enzymes. Proc. Natl. Acad. Sci. USA 2002, 99, 6661–6666. [Google Scholar] [CrossRef] [Green Version]
- Ninh, P.H.; Honda, K.; Yokohigashi, Y.; Okano, K.; Omasa, T.; Ohtake, H. Development of a Continuous Bioconversion System Using a Thermophilic Whole-Cell Biocatalyst. Appl. Environ. Microbiol. 2013, 79, 1996–2001. [Google Scholar] [CrossRef] [Green Version]
- Krumbein, W.E.; Altmann, H.J. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgoländer Wiss. Meeresunters. 1973, 25, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Hastings, D.; Emerson, S. Oxidation of manganese by spores of a marine bacillus: Kinetic and thermodynamic considerations. Geochim. et Cosmochim. Acta 1986, 50, 1819–1824. [Google Scholar] [CrossRef]
- Das, A.P.; Sukla, L.B.; Pradhan, N.; Nayak, S. Manganese biomining: A review. Bioresour. Technol. 2011, 102, 7381–7387. [Google Scholar] [CrossRef]
- Miyata, N.; Tani, Y.; Sakata, M.; Iwahori, K. Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 2007, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, S.; Tai, Y.; Tao, R.; Dai, Y.; Guo, J.; Yang, Y.; Duan, S. Biogenic manganese oxides generated by green algae Desmodesmus sp. WR1 to improve bisphenol A removal. J. Hazard. Mater. 2017, 339, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Spiro, T.G.; Bargar, J.R.; Sposito, G.; Tebo, B.M. Bacteriogenic Manganese Oxides. Acc. Chem. Res. 2010, 43, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Chen, N.; Ding, H.; Zhang, H.; Liu, F.; Yin, H.; Chu, S.; Wang, C.; Lu, A. Cu (II) sorption by biogenic birnessite produced by Pseudomonas putida strain MnB1: Structural differences from abiotic birnessite and its environmental implications. Cryst. Eng. Comm. 2018, 20, 1361–1374. [Google Scholar] [CrossRef]
- Forrez, I.; Carballa, M.; Verbeken, K.; Vanhaecke, L.; Ternes, T.; Boon, N.; Verstraete, W. Diclofenac Oxidation by Biogenic Manganese Oxides. Environ. Sci. Technol. 2010, 44, 3449–3454. [Google Scholar] [CrossRef]
- Florian, R. Whole-Cell based synthetic enzyme cascades-Light and shadow of a promising technology. Curr. Opin. Chem. Biol. 2019, 49, 84–90. [Google Scholar]
- Boogerd, F.C.; de Vrind, J.P. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 1987, 169, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Zhang, Y.; Xu, Z.-J.; Che, C.-M. Iron (III)–Salan complexes catalysed highly enantioselective fluorination and hydroxylation of β-keto esters and N-Boc oxindoles. Chem. Comm. 2014, 50, 7870–7873. [Google Scholar] [CrossRef]
- Boukouvalas, D.T.; Prates, R.A.; Leal, C.R.L.; de Araújo, S.A. Automatic segmentation method for CFU counting in single plate-Serial dilution. Chemom. Intell. Lab. Syst. 2019, 195, 103889. [Google Scholar] [CrossRef]




| Data | Dried BMO 1 | CMO 2 |
|---|---|---|
| Rate constant (k, h−1) | 0.9843 ± 0.0215 | 0.296 ± 0.0286 |
| R2 | 0.97~0.98 | 0.98~0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Guo, H.; Liu, J.; Zhong, F.; Wu, Y. Manganese(II) Oxidizing Bacteria as Whole-Cell Catalyst for β-Keto Ester Oxidation. Int. J. Mol. Sci. 2020, 21, 1709. https://doi.org/10.3390/ijms21051709
Guo J, Guo H, Liu J, Zhong F, Wu Y. Manganese(II) Oxidizing Bacteria as Whole-Cell Catalyst for β-Keto Ester Oxidation. International Journal of Molecular Sciences. 2020; 21(5):1709. https://doi.org/10.3390/ijms21051709
Chicago/Turabian StyleGuo, Juan, Huan Guo, Jin Liu, Fangrui Zhong, and Yuzhou Wu. 2020. "Manganese(II) Oxidizing Bacteria as Whole-Cell Catalyst for β-Keto Ester Oxidation" International Journal of Molecular Sciences 21, no. 5: 1709. https://doi.org/10.3390/ijms21051709