Involvement of Receptor for Advanced Glycation Endproducts in Hypertensive Disorders of Pregnancy
Abstract
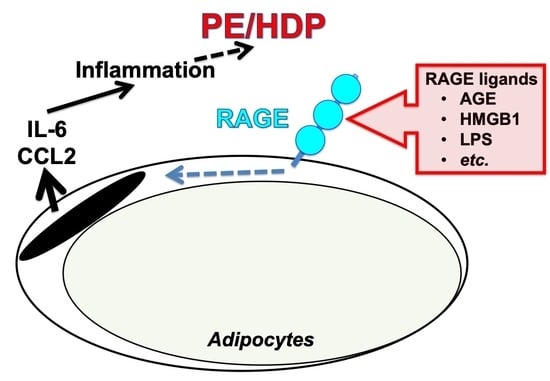
:1. Introduction
2. Results
2.1. PE/HDP Patient Sera Up-Regulated Gene Expression of IL-6, CCL2, High Mobility Group Box (HMGB)1, S100 Ca2+-Binding Protein B (S100B), and Receptor for Advanced Glycation Endproducts (RAGE) in Primary Cultured Human Adipocytes
2.2. Up-Regulation of IL-6 and CCL2 by HMGB1 and Advanced Glycation Endproducts (AGE) in Adipocytes
2.3. Down-Regulation of RAGE Attenuated the Increases of IL-6 and CCL2 in Adipocytes Treated with Small Interfering RNA (siRNA) for RAGE
2.4. Up-Regulation of IL-6 and CCL2 by Lipopolysaccharide (LPS) in Adipocytes
2.5. Down-Regulation of RAGE Attenuated the LPS-Induced IL-6 and CCL2 Increases in Adipocytes
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Cell Culture and Treatment
4.3. Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.4. Measurement of IL-6 and CCL2 Concentrations in Culture Medium via ELISA
4.5. RNA Interference (RNAi)
4.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADIP | Adiponectin |
| AGE | Advanced glycation endproduct(s) |
| BMI | Body mass index |
| CCL2 | C-C motif chemokine ligand 2 |
| ELISA | Enzyme-linked immunosorbent assay |
| esRAGE | Endogenous secretory RAGE |
| FCS | Fetal calf serum |
| HbA1c | Hemoglobin A1c |
| HMGB1 | High mobility group box 1 |
| IBMX | Methylisobutylxanthine |
| IL-6 | Interleukin-6 |
| LEP | Leptin |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear factor κ-light-chain-enhancer of activated B cells |
| PCR | Polymerase chain reaction |
| PE/HDP | Preeclampsia/hypertensive disorders of pregnancy |
| RAGE | Receptor for advanced glycation endproduct(s) |
| RETN | Resistin |
| Rig/RpS15 | Rat insulinoma gene/Ribosomal protein S15 |
| RT-PCR | Reverse transcriptase-PCR |
| S100B | S100 Ca2+-binding protein B |
| siRNA | Small interfering RNA |
| sRAGE | Soluble RAGE |
| TNFα | Tumor necrosis factor α |
References
- Walker, J.J. Pre-eclampsia. Lancet 2000, 356, 1260–1265. [Google Scholar] [CrossRef]
- Kintiraki, E.; Papakatsika, S.; Kotronis, G.; Goulis, D.G.; Kotsis, V. Pregnancy-induced hypertension. Hormones 2015, 14, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Freeman, D.J.; McManus, F.; Brown, E.A.; Cherry, L.; Norrie, J.; Ramsay, J.E.; Clark, P.; Walker, I.D.; Sattar, N.; Greer, I.A. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 2004, 44, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Von Versen-Hoeynck, F.M.; Powers, R.W. Maternal-fetal metabolism in normal pregnancy and preeclampsia. Front. Biosci. 2007, 12, 2457–2470. [Google Scholar] [CrossRef] [PubMed]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclamsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar]
- Brennan, L.J.; Morton, J.S.; Davidge, S.T. Vascular dysfunction in preeclampsia. Microcirculation 2014, 21, 4–14. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Li, H.; Chen, X.; Tong, X. Analysis of the original causes of placental oxidative stress in normal pregnancy and preeclampsia: a hypothesis. J. Matem. Fetal Neonatal Med. 2012, 25, 884–888. [Google Scholar] [CrossRef]
- Fisher, S.J. Why is placentation abnormal in preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Nakashima, A. A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion vascular remodeling. J. Reprod. Immunol. 2014, 101, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, W.W., Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalagiri, R.R.; Carder, T.; Choudhury, S.; Vora, N.; Ballard, A.R.; Govande, V.; Drever, N.; Beeram, M.R.; Uddin, M.N. Inflammation in complicated pregnancy and its outcome. Am. J. Perinatol. 2016, 33, 1337–1356. [Google Scholar] [CrossRef] [PubMed]
- Shamshirsaz, A.A.; Paidas, M.; Krikun, G. Preeclamsia, hypoxia, thrombosis, and inflammation. J. Pregnancy 2012, 2012, 374047. [Google Scholar] [CrossRef]
- Kim, Y.J. Pathogenesis and promising non-invasive markers for preeclampsia. Obstet. Gynecol. Sci. 2013, 56, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Shah, A.; Mehta, N.; Reilly, M.P. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J. Parenter. Enteral Nutr. 2008, 32, 638–644. [Google Scholar] [CrossRef]
- Bartha, J.L.; Marín-Segura, P.; González-González, N.L.; Wagner, F.; Aguilar-Diosdado, M.; Hervias-Vivancos, B. Ultrasound evaluation of visceral fat and metabolic risk factors during early pregnancy. Obesity 2007, 15, 2233–2239. [Google Scholar] [CrossRef]
- Taebi, M.; Sadat, Z.; Saberi, F.; Kalahroudi, M.A. Early pregnancy waist-to-hip ratio and risk P preeclampsia: a prospective cohort study. Hypertens. Res. 2015, 38, 80–83. [Google Scholar] [CrossRef]
- De Souza, L.R.; Kogan, E.; Berger, H.; Alves, J.G.; Lebovic, G.; Retnakaran, R.; Maguire, J.L.; Ray, J.G. Abdominal adiposity and insulin resistance in early pregnancy. J. Obstet. Gynaecol. Can. 2014, 36, 969–975. [Google Scholar] [CrossRef]
- Zhang, S.; Folsom, A.R.; Flack, J.M.; Liu, K. Body fat distribution before pregnancy and gestational diabetes: findings from coronary artery risk development in young adults (CARDIA) study. BMJ 1995, 311, 1139–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Babini, L.; Della Guardia, L. Potential role of microRNAs in the regulation of adipocytes liposecretion and adipose tissue physiology. J. Cell. Physiol. 2018, 233, 9077–9086. [Google Scholar] [CrossRef] [PubMed]
- Barden, A. Pre-eclampsia: contribution of maternal constitutional factors and the consequences for cardiovascular health. Clin. Exp. Pharmacol. Physiol. 2006, 33, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jaramillo, P.; Barajas, J.; Rueda-Quijano, S.M.; Lopez-Lopez, C.; Felix, C. Obesity and preeclampsia: Common pathophysiological mechanisms. Front. Physiol. 2018, 9, 1838. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diab. Rep. 2015, 15, 9. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Abell, A.M.; Moon, Y.S.; Kim, K.-H. An advanced glycation end product (AGE)-receptor for AGEs (RAGE) axis restores adipogenic potential of senescent preadipocytes through modulation of p53 protein function. J. Biol. Chem. 2012, 287, 44498–44507. [Google Scholar] [CrossRef]
- Nativel, R.; Marimoutou, M.; Thon-Hon, V.G.; Guanasekaran, M.K.; Andries, J.; Stanislas, G.; Da Silva, C.R.; Césari, M.; Iwema, T.; Gasque, P.; et al. Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: Contribution to chronic inflammation in fat tissue. PLoS ONE 2013, 8, e76039. [Google Scholar] [CrossRef]
- Tanaka, N.; Yonekura, H.; Yamagishi, S.; Fujimori, H.; Yamamoto, Y.; Yamamoto, H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-α through nuclear factor-κB, and by 17β-estradiol through Sp-1 in human vascular endothelial cells. J. Biol. Chem. 2000, 275, 25781–25790. [Google Scholar] [CrossRef]
- Fujiya, A.; Nagasaki, H.; Seino, Y.; Okawa, T.; Kato, J.; Fukami, A.; Himeno, T.; Uenishi, E.; Tsunekawa, S.; Kamiya, H.; et al. The role of S100B in the interaction between adipocytes and macrophages. Obesity 2014, 22, 371–379. [Google Scholar] [CrossRef]
- Son, K.H.; Son, M.; Ahn, H.; Oh, S.; Yum, Y.; Choi, C.H.; Park, K.Y.; Byun, K. Age-related accumulation of advanced glycation end-products-albumin, S100β, and the expressions of advanced glycation end product receptor differ in visceral and subcutaneous fat. Biochem. Biophys. Res. Commun. 2016, 477, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-Z.; Xiao, X.-M. Evaluation of the effects of Uncaria rhynchophylla alkaloid extract on LPS-induced preeclamsia symptoms and inflammation in pregnant rat model. Braz. J. Med. Biol. Res. 2019, 52, e8273. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Harashima, A.; Saito, H.; Tsuneyama, K.; Munesue, S.; Motoyoshi, S.; Han, D.; Watanabe, T.; Asano, M.; Takasawa, S.; et al. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J. Immunol. 2011, 186, 3248–3257. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, J.; Ribeiro, C.T.; Bortolin, R.C.; Somensi, N.; Fernandes, H.S.; Teixeira, A.A.; Guasselli, M.O.R.; Agani, C.A.J.O.; Souza, N.C.; Grings, M.; et al. Anti-RAGE antibody selectively blocks acute systemic inflammatory responses to LPS in serum, liver, CSF and striatum. Brain Behav. Immun. 2017, 62, 124–136. [Google Scholar] [CrossRef]
- Ramsgaard, L.; Englert, J.M.; Manni, M.L.; Milutinovic, P.S.; Gefter, J.; Tobolewski, J.; Crum, L.; Coudriet, G.M.; Piganelli, J.; Zamora, R.; et al. Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in mice. PLoS ONE 2011, 6, e20132. [Google Scholar] [CrossRef]
- Rineiro, C.T.; Gasparotto, J.; Teixeira, A.A.; Portela, L.V.C.; Flores, V.N.L.; Moreira, J.C.F.; Gelain, D.P. Immune neutralization of the receptor for advanced glycation end products reduce liver oxidative damage induced by an acute systemic injection of lipopolysaccharide. J. Biochem. 2018, 163, 515–523. [Google Scholar]
- Wang, L.; Wu, J.; Guo, X.; Huang, X.; Huang, Q. RAGE plays a role in LPS-induced NF-κB activation and endothelial hyperpermeability. Sensors 2017, 17, 722. [Google Scholar] [CrossRef]
- Li, Y.; Wu, R.; Zhao, S.; Cheng, H.; Ji, P.; Yu, M.; Tian, Z. RAGE/NF-κB pathway mediates lipopolysaccharide-induced inflammation in alveolar type I epithelial cells isolated from neonate rats. Inflammation 2014, 37, 1623–1629. [Google Scholar] [CrossRef]
- Umesawa, M.; Kobashi, G. Epidemiology of hypertensive disorders in pregnancy: Prevalence, risk factors, predictors and prognosis. Hypertens. Res. 2017, 40, 213–220. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Wang, L.; Qiu, M.; Wang, Y.; Hou, X.; Guo, Z.; Wang, B. Hypertensive disorders during pregnancy and risk of type 2 diabetes in later life: a systematic review and meta-analysis. Endocrine 2017, 55, 809–821. [Google Scholar] [CrossRef]
- Sohlberg, S.; Stephansson, O.; Cnattingius, S.; Wikström, A.-K. Maternal body mass index, height, and risks of preeclampsia. Am. J. Hypertens. 2012, 25, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.C.; Mahmood, T. Obesity in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lutsiv, O.; Mah, J.; Beyene, J.; McDonald, S.D. The effects of morbid obesity on maternal and neonatal health outcomes: a systematic review and meta-analyses. Obes. Rev. 2015, 16, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Mission, J.F.; Marshall, N.E.; Caughey, A.B. Pregnancy risks associated with obesity. Obstet. Gynecol. Clin. North Am. 2015, 42, 335–353. [Google Scholar] [CrossRef]
- Maclnnis, N.; Woolcott, C.G.; McDonald, S.; Kuhle, S. Population attributable risk fractions of maternal overweight and obesity for adverse perinatal outcomes. Sci. Rep. 2016, 6, 22895. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.; Dencker, A.; Olander, E.K.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef]
- Santangeli, L.; Sattar, N.; Huda, S.S. Impact of maternal obesity on perinatal and childhood outcomes. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 438–448. [Google Scholar] [CrossRef]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal obesity, inflammation, and developmental programming. BioMed Res. Int. 2014, 2014, 418975. [Google Scholar] [CrossRef]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl. Res. 2016, 167, 228–256. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M. Adiponectin: a versatile player of innate immunity. J. Mol. Cell Biol. 2016, 8, 120–128. [Google Scholar] [CrossRef]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Kulyté, A.; Belarbi, Y.; Lorente-Cebrián, S.; Bambace, C.; Arner, E.; Daub, C.O.; Hedén, P.; Rydén, M.; Mejhert, N.; Arner, P. Additive effects of microRNAs and transcription factors on CCL2 production in human white adipose tissue. Diabetes 2014, 63, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Sachs, U.J.; Chavakis, T.; Fung, L.; Lohrenz, A.; Bux, J.; Reil, A.; Ruf, A.; Santoso, S. Human alloantibody anti-Mart interferes with Mac-1-dependent leukocyte adhesion. Blood 2004, 104, 727–734. [Google Scholar] [CrossRef]
- Masuda, N.; Tsujinaka, H.; Hirai, H.; Yamashita, M.; Ueda, T.; Ogata, N. Effects of concentration of amyloid β (Aβ) on viability of cultured retinal pigment epithelial cells. BMC Ophthalmol. 2019, 19, 70. [Google Scholar] [CrossRef]
- Rong, L.L.; Gooch, C.; Szabolcs, M.; Herold, K.C.; Lalla, E.; Hays, A.P.; Yan, S.F.; Yan, S.S.; Schmidt, A.M. RAGE: a journey from the complications of diabetes to disorders of nervous system – striking a fine balance between injury and repair. Restor. Neurol. Neurosci. 2005, 23, 355–365. [Google Scholar]
- Zhang, Z.; Yang, L.; Lei, L.; Chen, R.; Chen, H.; Zhang, H. Glucagon-like peptide-1 attenuates advanced oxidation protein product-mediated damage in islet microvascular endothelial cells partly through the RAGE pathway. Int. J. Mol. Med. 2016, 38, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Ruan, B.H.; Li, X.; Winkler, A.R.; Cunningham, K.M.; Kuai, J.; Greco, R.M.; Nocka, K.H.; Fitz, L.J.; Wright, J.F.; Pittman, D.D.; et al. Complement C3a, CpG oligos, and DNA/C3a complex stimulate IFN-α production in a receptor for advanced glycation end product-dependent manner. J. Immunol. 2010, 185, 4213–4222. [Google Scholar] [CrossRef]
- He, M.; Kubo, H.; Morimoto, K.; Fujino, N.; Suzuki, T.; Takahasi, T.; Yamada, M.; Yamaya, M.; Maekawa, T.; Yamamoto, Y.; et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011, 12, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.d.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advance glycation end-products expressed in human vascular endothelial cells pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef]
- Harashima, A.; Yamamoto, Y.; Cheng, C.; Tsuneyama, K.; Myint, K.M.; Takeuchi, A.; Yoshimura, K.; Li, H.; Watanabe, T.; Takasawa, S.; et al. Identification of mouse orthologue of endogenous secretory receptor for advanced glycation end-products: structure, function and expression. Biochem. J. 2006, 396, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.-H.; Kim, Y.-H.; Kwon, J.-Y.; Park, Y.-W. Clinical significance of serum sRAGE and esRAGE in women with normal pregnancy and preeclampsia. J. Perinat. Med. 2011, 39, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.L.; Brockelsby, J.C.; Baker, P.N.; Davidge, S.T. The receptor for advanced glycation end products (RAGE) is elevated in women with preeclampsia. Hypertens. Pregnancy 2003, 22, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Hodge, D.R.; Wang, L.; Yang, X.; Zhang, X.; Farrar, W.L. Co-operative functions between nuclear factors NFκB and CCAT/enhancer-binding protein-β (C/EBP-β) regulate the IL-6 promoter in autocrine human prostate cancer cells. Prostate 2004, 61, 354–370. [Google Scholar] [CrossRef]
- Rajaiya, J.; Sadeghi, N.; Chodosh, J. Specific NFκB subunit activation and kinetics of cytokine induction in adenoviral keratitis. Mol. Vis. 2009, 15, 2879–2889. [Google Scholar]
- Kay, A.M.; Simpson, C.L.; Stewart, J., Jr. A. The role of AGE/RAGE signaling in diabetes–mediated vascular calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef]
- Naruse, K.; Akasaka, J.; Shigemitsu, A.; Tsunemi, T.; Koike, N.; Yoshimoto, C.; Kobayashi, H. Involvement of visceral adipose tissue in immunological modulation of inflammatory cascade in preeclampsia. Mediators Inflamm. 2015, 2015, 325932. [Google Scholar] [CrossRef]
- Uchiyama, T.; Ota, H.; Itaya-Hironaka, A.; Shobatake, R.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Makino, M.; Kimura, H.; Takeda, M.; Ohbayashi, C.; et al. Up-regulation of selenoprotein P and HIP/PAP mRNAs in hepatocytes by intermittent hypoxia via down-regulation of miR-203. Biochem. Biophys. Rep. 2017, 11, 130–137. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Buhrow, S.A.; Kaestner, K.H.; Christy, R.J.; Sibley, E.; Kelly Jr., T.J.; Lane, M.D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J. Biol. Chem. 1988, 263, 17291–17300. [Google Scholar]
- Uchiyama, T.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Shobatake, R.; Ota, H.; Takeda, M.; Ohbayashi, C.; Takasawa, S. Intermittent hypoxia up-regulates CCL2, RETN, and TNFα mRNAs in adipocytes via down-regulation of miR-452. Int. J. Mol. Sci. 2019, 20, 1960. [Google Scholar] [CrossRef]
- Ota, H.; Tamaki, S.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Morioka, T.; Takasawa, S.; Kimura, H. Attenuation of glucose-induced insulin secretion by intermittent hypoxia via down-regulation of CD38. Life Sci. 2012, 90, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Miyaoka, T.; Fujimura, T.; Tsujinaka, H.; Yoshimoto, K.; Nakagawara, K.; Tamaki, S.; et al. Pancreatic β cell proliferation by intermittent hypoxia via up-regulation of Reg family genes and HGF gene. Life Sci. 2013, 93, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Takasawa, S.; Nata, K.; Yamauchi, A.; Itaya-Hironaka, A.; Ota, H.; Yoshimoto, K.; Sakuramoto-Tsuchida, S.; Miyaoka, T.; Takeda, M.; et al. Prevention of Reg I-induced β-cell apoptosis by IL-6/dexamethasone through activation of HGF gene regulation. Biochim Biophys Acta 2013, 1833, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Takeda, M.; Yoshimoto, K.; Miyaoka, T.; Fujimura, T.; Tsujinaka, H.; Tsuchida, C.; Ota, H.; et al. Synergistic activations of REG Iα and REG Iβ promoters by IL-6 and glucocorticoids through JAK/STAT pathway in human pancreatic β cells. J. Diabetes Res. 2015, 2015, 173058. [Google Scholar] [CrossRef]
- Fujimura, T.; Fujimoto, T.; Itaya-Hironaka, A.; Miyaoka, T.; Yoshimoto, K.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Kondo, S.; Takeda, M.; Tsujinaka, H.; et al. Interleukin-6/STAT pathway is responsible for the induction of gene expression of REG Iα, a new auto-antigen in Sjögren’s syndrome patients, in salivary duct epithelial cells. Biochem. Biophys. Rep. 2015, 2, 69–74. [Google Scholar] [CrossRef]
- Tsujinaka, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Ota, H.; Takeda, M.; Fujimura, T.; Takasawa, S.; Ogata, N. Human retinal pigment epithelial cell proliferation by the combined stimulation of hydroquinone and advanced glycation end-products via up-regulation of VEGF gene. Biochem. Biophys. Rep. 2015, 2, 123–131. [Google Scholar] [CrossRef]
- Tsuchida, C.; Sakuramoto-Tsuchida, S.; Takeda, M.; Itaya-Hironaka, A.; Yamauchi, A.; Misu, M.; Shobatake, R.; Uchiyama, T.; Makino, M.; Pujol-Autonell, I.; et al. Expression of REG family genes in human inflammatory bowel diseases and its regulation. Biochem. Biophys. Rep. 2017, 12, 198–205. [Google Scholar] [CrossRef]
- Tsujinaka, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Shobatake, R.; Makino, M.; Masuda, N.; Hirai, H.; Takasawa, S.; Ogata, N. Statins decrease vascular epithelial growth factor expression via down-regulation of receptor for advanced glycation end-products. Heliyon 2017, 3, e00401. [Google Scholar] [CrossRef]
- Tohma, Y.; Dohi, Y.; Shobatake, R.; Uchiyama, T.; Takeda, M.; Takasawa, S.; Tanaka, Y.; Ohgushi, H. Reg gene expression in periosteum after fracture and its in vitro induction triggered by IL-6. Int. J. Mol. Sci. 2017, 18, 2257. [Google Scholar] [CrossRef]
- Shobatake, R.; Takasawa, K.; Ota, H.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Makino, M.; Sugie, K.; Takasawa, S.; et al. Up-regulation of POMC and CART mRNAs by intermittent hypoxia via GATA transcription factors in human neuronal cells. Int. J. Biochem. Cell Biol. 2018, 95, 100–107. [Google Scholar] [CrossRef]
- Kyotani, Y.; Itaya-Hironaka, A.; Yamauchi, A.; Sakuramoto-Tsuchida, S.; Makino, M.; Takasawa, S.; Yoshizumi, M. Intermittent hypoxia-induced epiregulin expression by IL-6 production in human coronary artery smooth muscle cells. FEBS Open Bio 2018, 8, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, S.; Tsuchida, C.; Sakuramoto-Tsuchida, S.; Takeda, M.; Itaya-Hironaka, A.; Yamauchi, A.; Misu, M.; Shobatake, R.; Uchiyama, T.; Makino, M.; et al. Expression of human REG family genes in inflammatory bowel disease and their molecular mechanism. Immunol. Res. 2018, 66, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Fujimoto, T.; Itaya-Hironaka, A.; Miyaoka, T.; Sakuramoto-Tsuchida, S.; Yamauchi, A.; Takeda, M.; Kasai, T.; Nakagawara, K.; Nonomura, A.; et al. Involvement of autoimmunity to REG, a regeneration factor, in patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2013, 174, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Murakami-Kawaguchi, S.; Takasawa, S.; Onogawa, T.; Nata, K.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Yamauchi, A.; Ota, H.; Takeda, M.; Kato, M.; et al. Expression of Ins1 and Ins2 genes in mouse fetal liver. Cell Tissue Res. 2014, 355, 303–314. [Google Scholar] [CrossRef]
- Shobatake, R.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Uchiyama, T.; Ota, H.; Takahashi, N.; Ueno, S.; Sugie, K.; et al. Intermittent hypoxia up-regulates gene expressions of peptide YY (PYY), glucagon-like peptide-1 (GLP-1), and neurotensin (NTS) in enteroendocrine cells. Int. J. Mol. Sci. 2019, 20, 1849. [Google Scholar] [CrossRef]









| Patients/Controls | Age (Years) | BMI | Gestational Age at Blood Sampling (Week) | Parity |
|---|---|---|---|---|
| PE/HDP #1 | 33 | 23.2 | 30 | 0 |
| PE/HDP #2 | 27 | 21.9 | 29 | 1 |
| PE/HDP #3 | 28 | 21.3 | 28 | 0 |
| PE/HDP #4 | 29 | 23.4 | 27 | 0 |
| Control #1 | 30 | 22.4 | 28 | 0 |
| Control #2 | 29 | 24.6 | 28 | 0 |
| Control #3 | 26 | 23.8 | 27 | 0 |
| Control #4 | 33 | 22.4 | 28 | 2 |
| Target mRNA | Primer Sequence (Position) |
|---|---|
| Human IL-6 (NM_000600) | 5′-GGTACATCCTCGACGGCATC-3′ (289–308) 5′- GCCTCTTTGCTGCTTTCACAC-3′ (347–367) |
| Human CCL2 (NM_002982) | 5′-GTCTCTGCCGCCCTTCTGT-3′ (80–98) 5′-TTGCATCTGGCTGAGCGAG-3′ (137–155) |
| Human TNFα (NM_000594) | 5′-CTTCTCCTTCCTGATCGTGG-3′ (280–299) 5′-TCTCAGCTCCACGCCATT-3′ (518–535) |
| Human LEP (NM_000230) | 5′-GGCTTTGGCCCTATCTTTTC-3′ (89–108) 5′-GGATAAGGTCAGGATGGGGT-3′ (257–276) |
| Human ADIP (NM_001177800) | 5′-CATGACCAGGAAACCACGACT-3′ (181–201) 5′-TGAATGCTGAGCGGTAT-3′ (465–481) |
| Human RETN (NM_020415) | 5′-TCCTCCTCCTCCCTGTCCTGG-3′ (63–83) 5′-CAGTGACATGTGGTCTGGGCG-3′ (298-318) |
| Human S100B (NM_006272) | 5′-AGGGAGGGAGACAAGCACAA-3′ (172–191) 5′-ACTCGTGGCAGGCAGTAGTA-3′ (293–312) |
| Human HMGB1 (NM_001313893) | 5′-ATATGGCAAAAGCGGACAAG-3′ (1126–1145) 5′-AGGCCAGGATGTTCTCCTTT-3′ (1281–1300) |
| Human RAGE (NM_001136) | 5′-TGGAACCGTAACCCTGACCT-3′ (856–875) 5′-CGATGATGCTGATGCTGACA-3′ (1045–1064) |
| Human β-actin (NM_001101) | 5′-GCGAGAAGATGACCCAGA-3’ (420–437) 5´-CAGAGGCGTACAGGGATA-3´ (492–509 |
| Mouse IL-6 (NM_031168) | 5′-GTATGAACAACGATGATGCACTTG-3′ (305–328) 5′-ATGGTACTCCAGAAGACCAGAGGA-3′ (418–441) |
| Mouse Ccl2 (NM_011333) | 5′-CCACTCACCTGCTGCTACTCAT-3′ (176–197) 5′-TGGTGATCCTCTTGTAGCTCTCC-3′ (229–251) |
| Mouse Rig/RpS15 (NM_009091) | 5′-ACGGCAAGACCTTCAACCAG-3′ (323–342) 5′-ATGGAGAACTCGCCCAGGTAG-3′ (372–392) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akasaka, J.; Naruse, K.; Sado, T.; Uchiyama, T.; Makino, M.; Yamauchi, A.; Ota, H.; Sakuramoto-Tsuchida, S.; Itaya-Hironaka, A.; Takasawa, S.; et al. Involvement of Receptor for Advanced Glycation Endproducts in Hypertensive Disorders of Pregnancy. Int. J. Mol. Sci. 2019, 20, 5462. https://doi.org/10.3390/ijms20215462
Akasaka J, Naruse K, Sado T, Uchiyama T, Makino M, Yamauchi A, Ota H, Sakuramoto-Tsuchida S, Itaya-Hironaka A, Takasawa S, et al. Involvement of Receptor for Advanced Glycation Endproducts in Hypertensive Disorders of Pregnancy. International Journal of Molecular Sciences. 2019; 20(21):5462. https://doi.org/10.3390/ijms20215462
Chicago/Turabian StyleAkasaka, Juria, Katsuhiko Naruse, Toshiyuki Sado, Tomoko Uchiyama, Mai Makino, Akiyo Yamauchi, Hiroyo Ota, Sumiyo Sakuramoto-Tsuchida, Asako Itaya-Hironaka, Shin Takasawa, and et al. 2019. "Involvement of Receptor for Advanced Glycation Endproducts in Hypertensive Disorders of Pregnancy" International Journal of Molecular Sciences 20, no. 21: 5462. https://doi.org/10.3390/ijms20215462