Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases
Abstract
:1. Bone Modeling and Remodeling
2. Mechanism of Bone Resorption Process
3. Reactive Oxygen Species (ROS)
3.1. Mitochondrial ROS
3.2. NADPH Oxidase
3.3. Hydrogen Peroxide
4. ROS in OC Differentiation and Activity
4.1. mtROS and OCs
4.2. NOX1 in OCs
4.3. NOX2 in OCs
4.4. NOX4 in OCs
4.5. H2O2 and Other ROS in OCs
5. Regulation of OC Differentiation by Redox Modulation
6. ROS in Signal Cascades of Osteoclastogenesis
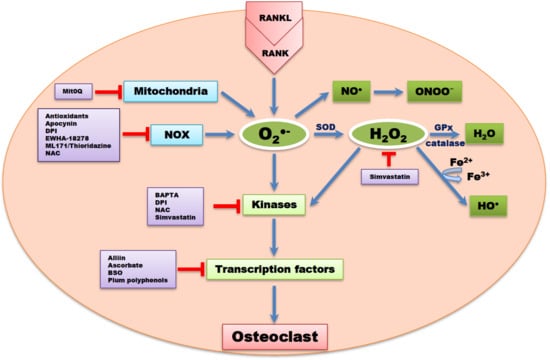
7. ROS as Pharmacological Targets for OC Associated Diseases
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT/PKB | protein kinase B |
| ATP | adenosine triphosphate |
| BAPTA | 1,2 bis(2-aminophenoxy) ethane-N, N, N′, N′-tetra acetic acid |
| BSP | bone sialoprotein |
| BMM | bone marrow macrophage |
| DPI | diphenyleneidonium |
| ER | endoplasmic reticulum |
| EGCG | epigallocatechin gallate |
| ERK | extracellular-signal-regulated kinase |
| HMG-CoA | 3-hydroxy-3 methylglutaryl coenzyme A |
| PB | peripheral blood |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated associated protein kinase |
| MCS | mesenchymal stem cell |
| M-CSF | macrophage colony stimulating factor |
| MMP | matrix metalloproteinase |
| mtROS | mitochondrial ROS |
| NFATc1 | nuclear factor of activated T cells 1 |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NF-κB | nuclear factor of kappa B |
| NAC | N-acetyl cysteine |
| NRROS | negative regulator of ROS |
| OB | osteoblast |
| OC | osteoclast |
| RANK | receptor activator of nuclear factor kappa B |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TNF | tumor necrotic factor |
| TRAF6 | TNF receptor associated factor 6 |
| TRAP | tartrate resistant acid phosphatase |
References
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [PubMed]
- Capulli, M.; Paone, R.; Rucc, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, M.B.; Cheung, W.Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Kramer, I.; Huber, T.; Kinzel, B.; Guth, G.S.; Leupin, O.; Kneissel, M. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc. Natl. Acad. Sci. 2014, 111, E5187–E5195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcoline, F.V.; Ishida, Y.; Mindell, J.A.; Nayak, S.; Grabe, M. A mathematical model of osteoclast acidification during bone resorption. Bone 2016, 93, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Zhu, S.; Ehnert, S.; Roub, M.; Häussling, V.; Aspera, R.H.; Chen, T.; Nussler, A.K. From the clinical problem to the basic research: co-culture models of osteoblasts and osteoclasts. Int. J. Mol. Sci. 2018, 19, 2284. [Google Scholar] [CrossRef]
- Sims, N.A.; Martin, T.J. Coupling signals between the osteoclast and osteoblast: how are messages transmitted between these temporary visitors to the bone surface? Front. Endocrinol. 2015, 6. [Google Scholar] [CrossRef]
- Lee, N.K. Molecular understanding of osteoclast differentiation and physiology. Endocrinol. Metab. 2010, 25, 264–269. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Nakashima, T.; Takayanagi, H. Osteocyte control of osteoclastogenesis. Bone 2013, 54, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of bone resorption in periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Plotkin, L.I.; Bruzzaniti, A. Bone cells. In Basic and Applied Bone Biology, 1st ed.; Burr, D., Allen, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 27–45. [Google Scholar]
- Stolina, M.; Dwyer, D.; Niu, Q.T.; Villasenor, K.S.; Kurimoto, P.; Grisanti, M.; Han, C.Y.; Liu, M.; Li, X.; Ominsky, M.S.; et al. Temporal changes in systemic and local expression of bone turnover markers during six months of sclerostin antibody administration to ovariectomized rats. Bone 2014, 67, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Diekman, B.O.; Leoser, R.F. Targeting aging for disease modification in osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Redox theory of aging: implications for health and disease. Clin. Sci. 2017, 131, 1669–1688. [Google Scholar] [CrossRef] [Green Version]
- Altindag, O.; Erel, O.; Soran, N.; Celik, H.; Selek, S. Total oxidative/anti-oxidative status and relation to bone mineral density in osteoporosis. Rheumatol. Int. 2008, 28, 317–321. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Schröder, K. NADPH oxidases in bone homeostasis and osteoporosis. Free. Radic. Biol. Med. 2019, 132, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, Y.; Ito, J.; Kitano, V.J.; Shimada, J.; Hakeda, Y. The polymethoxy flavonoid sudachitin suppresses inflammatory bone destruction by directly inhibiting osteoclastogenesis due to reduced ROS production and MAPK activation in osteoclast precursors. PLoS ONE 2018, 13, e0191192. [Google Scholar] [CrossRef] [PubMed]
- Bongio, M.; Lopa, S.; Gilardi, M.; Bersini, S.; Moretti, M. A 3D vascularized bone remodeling model combining osteoblasts and osteoclasts in a CaP nanoparticle-enriched matrix. Nanomed. 2016, 11, 1073–1091. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Yamashita, A.; Niimi, S.; Yamazaki, T. Involvement of reactive oxygen species in osteoblastic differentiation of MC3T3-E1 cells accompanied by mitochondrial morphological dynamics. Biomed. Res. 2013, 34, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maria, S.; Swanson, M.H.; Enderby, L.T.; D’amico, F.; Enderby, B.; Samsonraj, R.M.; Dudakovic, A.; van Wijnen, A.J.; Witt-Enderby, P.A. Melatonin-micronutrients osteopenia treatment study (MOTS): a translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging 2017, 9, 256–285. [Google Scholar]
- Valdes, A.M.; Goldring, M.B. Mitochondrial DNA haplogroups and ageing mechanisms in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.M.; Soto, H.A.; Vázquezmosquera, M.E.; Cortés, P.E.; Relaño, S.; Hermida, G.T.; Pértega, S.; Oreiro, V.N.; Fernández, L.C.; Garesse, R.; et al. Mitochondrial DNA haplogroups influence the risk of incident knee osteoarthritis in OAI and CHECK cohorts. A meta-analysis and functional study. Ann. Rheum. Dis. 2017, 76, 1114–1122. [Google Scholar]
- Li, X.; Fang, P.; Mai, J.; Chai, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef]
- Lasségue, B.; SanMartin, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar] [CrossRef]
- Qiu, Y.; Tao, L.; Lei, C.; Wang, J.; Yang, P.; Li, Q.; Lei, B. Downregulating p22phox ameliorates inflammatory response in angiotensin II-induced oxidative stress by regulating MAPK and NF-κB pathways in ARPE-19 cells. Sci. Rep. 2015, 5, 14362. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.F.; Peng, Y.B.; Hu, Y.P.; Shi, Y.Z.; Yao, M.; Zhang, X. NADPH oxidase 1 and its derived reactive oxygen species mediated tissue injury and repair. Oxid. Med. Cell. Longev. 2014, 2014, 282854. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, F.R.; Araujo, T.L.; Abrahão, T.B. Nox NADPH oxidase and the endoplasmic reticulum. Antioxid. Redox Signal. 2014, 20, 2755–2775. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell Biol. 2004, 122, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Hejmo, T.; Poterala, H.A.; Cieslara, P.A.; Bulkad, R.J. NADPH oxidases: Insights into selected functions and mechanisms of action in cancer and stem cells. Oxid. Med. Cell Longev. 2017, 2017, 9420539. [Google Scholar] [CrossRef]
- Ueno, N.; Takeya, R.; Miyano, K.; Kikuchi, H.; Sumimoto, H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J. Biol. Chem. 2005, 280, 23328–23339. [Google Scholar] [CrossRef]
- Kuroda, J.; Nakagawa, K.; Yamasaki, T.; Nakamura, K.; Takeya, R.; Kuribayashi, F.; Imajoh, O.S.; Igarashi, K.; Shibata, Y.; Sueishi, K.; et al. The superoxide producing NADPH oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 2005, 10, 1139–1151. [Google Scholar] [CrossRef]
- Rastogi, R.; Geng, X.; Li, F.; Ding, Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front. Cell Neurosci. 2017, 10, 301. [Google Scholar] [CrossRef]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: a hydrogen peroxide generating oxygen sensor. Biochemistry 2014, 53, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar]
- Harrison, C. Bone disorders: targeting NOX4 knocks down osteoporosis. Nat. Rev. Drug Discov. 2013, 12, 904–905. [Google Scholar] [CrossRef] [PubMed]
- Burtenshaw, D.; Hakimjavadi, R.; Redmond, E.M.; Cahill, P.A. Nox, reactive oxygen species and regulation of vascular cell fate. Antioxidants 2017, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Jaquet, V.; Krause, K.H. NOX5: from basic biology to signaling and disease. Free Radic. Biol. Med. 2012, 52, 725–734. [Google Scholar] [CrossRef]
- Montezano, A.C.; De Lucca, C.L.; Persson, P.; Rios, F.J.; Harvey, A.P.; Anagnostopoulou, A.; Palacios, R.; Gandara, A.C.; Alves, L.R.; Neves, K.B.; et al. NADPH oxidase 5 is pro-contractile Nox isoform and a point of cross-talk for calcium and redox signaling implications in vascular function. J. Am. Heart Assoc. 2018, 7, e009388. [Google Scholar] [CrossRef] [PubMed]
- Carvalhoa, D.P.; Dupuy, C. Role of the NADPH oxidases DUOX and NOX4 in thyroid oxidative stress. Eur. Thyroid J. 2013, 2, 160–167. [Google Scholar] [CrossRef]
- Aprioku, J.A. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J. Reprod. Infertil. 2013, 14, 158–172. [Google Scholar]
- Rodiñojaneiro, B.K.; Paradela, D.B.; Castiñeiras, M.I.; Raposeirs-Rubin, S.; González-Juanatey, J.R.; Alvarez, E. Current status of NADPH oxidase research in cardiovascular pharmacology. Vasc. Health Risk Manag. 2013, 9, 401–428. [Google Scholar]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB mediated regulation of osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 23, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.J. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia 2015, 3, 73–82. [Google Scholar] [CrossRef]
- Srinivasa, S.; Koenigstein, A.; Joseph, J.; Sun, L.; Kalyanaraman, B.; Zaidi, M.; Avadhani, N.G. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann. N.Y. Acad. Sci. 2010, 1192, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Morten, K.J.; Badder, L.; Knowles, H.J. Differential regulation of HIF-mediated pathways increase mitochondrial metabolism and ATP production in hypoxic osteoclasts. J. Pathol. 2013, 229, 755–764. [Google Scholar] [CrossRef]
- Guha, M.; Srinivasan, S.; Koenigstein, A.; Zaidi, M.; Avadhani, N.G. Enhanced osteoclastogenesis by mitochondrial retrograde signaling through transcriptional activation of the cathepsin K gene. Ann. N.Y. Acad. Sci. 2016, 1364, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.K.; Jang, H.D. Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging. Int. J. Mol. Sci. 2014, 15, 10605–10621. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Choksi, S.; Qu, J.; Jang, J.; Choe, M.; Banfi, B.; Engelhardt, J.F.; Liu, Z.G. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 2016, 291, 20030–20041. [Google Scholar] [CrossRef]
- Kang, I.S.; Kim, C. NADPH oxidase gp91phox contributes to RANKL-induced osteoclast differentiation by upregulating NFATc1. Sci. Rep. 2016, 6, 38014. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, K.; Kim, I.; Seong, S.; Kim, N. NRROS negatively regulates osteoclast differentiation by inhibiting RANKL mediated NF-κB and reactive oxygen species pathways. Mol. Cells 2015, 38, 904–910. [Google Scholar]
- Yang, S.; Zhang, Y.; Ries, W.; Key, L. Expression of Nox4 in osteoclasts. J. Cell. Biochem. 2004, 92, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Madyastha, P.; Bingel, S.; Ries, W.; Key, L. A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 2001, 276, 5452–5458. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Babelova, A.; Trummer, O.; Erben, R.G.; Rauner, M.; Rammelt, S.; Weissmann, N.; Weinberger, V.; Benkhoff, S.; Kampschulte, M.; et al. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Invest. 2013, 123, 4731–4738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, F.; Rousset, F.; Vuchuong, N.M.; Trocme, C.; Grange, L.; Lardy, B. NADPH oxidase Nox4, a putative therapeutic target in osteoarthritis. Bull. Acad. Natl. Med. 2015, 199, 673–686. [Google Scholar] [PubMed]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska, K.K.; Woja, U. Oxidant/antioxidant imbalance in Alzheimer’s disease: Therapeutic and diagnostic prospects. Oxid. Med. Cell. Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant properties dedicated to nanotechnologies. Antioxidants. 2018, 7, 62. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the potential role of glutathione in multiple forms of cell death in cancer therapy. Oxid. Med. Cell Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.; Leanna, J.S.; Noel, S.W.; Jeannie, M.P.; Terrance, J.K.; Collin, C.W.; Michael, E.R. Glutathione as a biomarker in Parkinson’s disease: associations with aging and disease severity. Oxid. Med. Cell. Longev. 2016, 2016, 9409363. [Google Scholar] [CrossRef]
- Huh, Y.J.; Kim, J.M.; Kim, H.; Song, H.; So, H.; Lee, S.Y.; Kwon, S.B.; Kim, H.J.; Kim, H.H.; Lee, S.H.; et al. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factor. Cell Death Differ. 2006, 13, 1138–1146. [Google Scholar] [CrossRef]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen deficiency bone loss. J. Clin. Invest. 2003, 112, 915–923. [Google Scholar] [CrossRef]
- Kim, H.; Kim, I.Y.; Lee, S.Y.; Jeong, D. Bimodal actions of reactive oxygen species in the differentiation and bone-resorbing functions of osteoclasts. FEBS Lett. 2006, 580, 5661–5665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.M.; Kim, H.H.; Lee, Z.H. Reactive oxygen species mediated RANK signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Agidigbi, T.S.; Kang, I.S.; Kim, C. Enhancement of RAW 264.7 cell differentiation into osteoclast by PD98059 involves glutathione synthesis. In Proceedings of the 9th Biennial Meeting of Society for Free Radical Research-Asia, Kyoto, Japan, 4–7 April 2019. No: T000098. [Google Scholar]
- Fujita, H.; Ochi, M.; Ono, M.; Aoyama, E.; Ogino, T.; Kondo, Y.; Ohuchi, H. Glutathione accelerates osteoclast differentiation and inflammatory bone destruction. Free Radic. Res. 2019, 53, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, S.; Lee, H.; Yang, Y.; Jeong, W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013, 65, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, Y.; Dusting, G.J. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011, 63, 218–242. [Google Scholar] [CrossRef]
- Hirotani, H.; Tuohy, N.A.; Woo, J.T.; Stern, P.H.; Clipstone, N.A. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW 264.7 cells. J. Biol. Chem. 2004, 279, 13984–13992. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, H.K.; Kim, D.K.; Lee, S.Y. Rac1 GTPase regulates osteoclast differentiation through TRANCE-induced NF kappa B activation. Mol. Cell. Biochem. 2006, 281, 55–61. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.M.; Lee, S.H.; Fisher, D.E.; Kook, H.; Kim, K.K.; Choi, Y.; Kim, N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine mediated osteoclastogenesis. J. Biol. Chem. 2005, 280, 35209–35216. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Altenhöfer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of NADPH oxidase inhibitors: selectivity & mechanisms for target engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [PubMed]
- Joo, J.H.; Huh, J.E.; Lee, J.H.; Park, D.R.; Lee, Y.; Lee, S.G.; Choi, S.; Lee, H.J.; Song, S.W.; Jeong, Y.; et al. A novel pyrazole derivative protects from ovariectomy-induced osteoporosis through the inhibition of NADPH oxidase. Sci. Rep. 2016, 6, 22389. [Google Scholar] [CrossRef] [PubMed]
- Seredenina, T.; Chiriano, G.; Filippova, A.; Nayernia, Z.; Mahiout, Z.; Fioraso-Cartier, L.; Plastre, O.; Scapozza, L.; Krause, K.H.; Jaquet, V. A subset of N-substituted phenothiazines inhibits NADPH oxidases. Free Radic. Biol. Med. 2015, 86, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bu, S.Y.; Lerner, M.; Stoecker, B.J.; Boldrin, E.; Brackett, D.J.; Lucas, E.A.; Smith, B.J. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif. Tissue Int. 2008, 82, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Kim, S.E.; Yun, Y.P.; Hwang, Y.S.; Bang, J.B.; Park, J.H.; Kwon, I.K. Simvastatin inhibits osteoclast differentiation by scavenging reactive oxygen species. Exp. Mol. Med. 2011, 43, 605–612. [Google Scholar] [CrossRef]
- Chung, Y.S.; Lee, M.D.; Lee, S.K.; Kim, H.M.; Fitzpatrick, L.A. HMG-CoA reductase inhibitors increase BMD in type 2 diabetes mellitus patients. J. Clin. Endocrinol. Metab. 2000, 85, 1137–1142. [Google Scholar]
- Schildknecht, S.; Weber, A.; Gerding, H.R.; Pape, R.; Robotta, M.; Drescher, M.; Marquardt, A.; Daiber, A.; Ferger, B.; Leist, M. The NOX1/4 inhibitor GKT136901 as selective and direct scavenger of peroxynitrite. Curr. Med. Chem. 2014, 21, 365–376. [Google Scholar] [CrossRef]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.Y.; Bruno, F.; Thakur, S.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; Abboud, H.E. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Renal Physiol. 2015, 308, 1276–1287. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Dou, C.; Li, N.; Kang, F.; Wang, Y.; Cao, Z.; Yang, X.; Dong, S. Alliin attenuated RANKL-induced osteoclastogenesis by scavenging reactive oxygen species through inhibiting Nox1. Int. J. Mol. Sci. 2016, 17, 1516. [Google Scholar] [CrossRef]
- Katsumata, Y.; Kanzak, H.; Honda, Y.; Yoshitomo, H.; Tanaka, T.; Yamaguchi, Y.; Kanako, I.; Sari, F.; Yutaka, M.; Tsuyoshi, N.; et al. Single local injection of epigallocatechin gallate-modified gelatin attenuates bone resorption and orthodontic tooth movement in mice. Polymers 2018, 10, 1384. [Google Scholar] [CrossRef]
- Vali, B.; Rao, L.; El-Sohemy, A. Epigallocathechin-3-gallate (EGCG) increases the formation of mineralized bone nodules by human osteoblast-like cells. J. Nutr. Biochem. 2007, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Pang, E.K.; Kim, C.S.; Yoo, Y.J.; Cho, K.S.; Chai, J.K.; Kim, C.K.; Choi, S.H. Inhibitory effects of green tea polyphenol (-) epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J. Periodontal Res. 2004, 39, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.G.; Mackinnon, E.S.; Josse, R.G.; Murray, T.M.; Strauss, A.; Rao, A.V. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporosis Int. 2007, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, E.S.; Rao, A.V.; Josse, R.G.; Rao, L.G. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type I collagen in postmenopausal women. Osteoporosis Int. 2011, 22, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Winyard, P.G. Dietary antioxidants in inflammatory arthritis: do they have any role in etiology or therapy? Nat. Clin. Pract. Rheumatol. 2008, 4, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.W.; Chen, C.Y.; Su, F.C.; Tu, Y.K.; Tsai, T.T.; Lin, C.F.; Jou, I.M. Reactive oxygen species are required for zoledronic acid-induced apoptosis in osteoclast precursors and mature osteoclast-like cells. Sci. Rep. 2017, 7, 44245. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Hauschka, P.V.; Tuan, R.S.; Steinbeck, M.J. Exposure to particles stimulates superoxide production by human THP-1 macrophages and avian HD-11EM osteoclasts activated by tumor necrosis factor-α and PMA. J. Arthroplasty 2002, 17, 335–346. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakaguchi, N.; Hachiya, M.; Nakayama, F.; Yamakawa, M.; Akashi, M. Role of catalase in monocytic differentiation of U937 cells by TPA: hydrogen peroxide as a second messenger. Leukemia 2009, 23, 761–769. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Shih, Y.R.; Kuo, T.K.; Lee, O.K.; Wei, Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008, 26, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Mercer, K.E.; Sims, C.R.; Yang, C.S.; Wynne, R.A.; Moutos, C.; Hogue, W.R.; Lumpkin, C.K.; Suva, L.J.; Chen, J.R.; Badger, T.M.; et al. Loss of functional NADPH oxidase 2 protects against alcohol induced bone resorption in female p47phox-/- mice. Alcohol Clin. Exp. Res. 2014, 38, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, C.K.; Lee, K.U.; Park, J.Y.; Cho, K.J.; Cho, Y.S.; Lee, H.R.; Moon, S.H.; Moon, H.B.; Yoo, B. Alpha-lipoic acid suppresses the development of collagen-induced arthritis and protects against bone destruction in mice. Rheumatol. Int. 2007, 27, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Shinohara, F.; Kanako, I.; Yamaguchi, Y.; Fukaya, S.; Miyamoto, Y.; Wada, S.; Nakamura, Y. Molecular regulatory mechanisms of osteoclastogenesis through cytoprotective enzymes. Redox Biol. 2016, 8, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Ming, L.; Shang, F.; Shen, L.; Chen, J.; Jin, Y. Apocynin suppression of NADPH oxidase reverses the aging process in mesenchymal stem cells to promote osteogenesis and increase bone mass. Sci. Rep. 2015, 5, 18572. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Choi, S.H.; Han, M.H.; Kim, G.Y.; Park, C.; Hong, S.H.; Lee, B.J.; Park, E.K.; Kim, S.O.; Leem, S.H.; et al. Protective effects of fermented oyster extract against RANKL-induced osteoclastogenesis through scavenging ROS generation in RAW 264.7 cells. Int. J. Mol. Sci. 2019, 20, 1439. [Google Scholar] [CrossRef]


| ROS | Cell Type | References |
|---|---|---|
| O2− | RAW 264.7 | [61,80,100] |
| mBMM | [80,100] | |
| HD-11EM | [101] | |
| HL-60 | [100,102] | |
| hBMM | [103] | |
| hMSC | [104] | |
| THP-1 | [100] | |
| U937 | [102] | |
| HP100-1 | [102] | |
| H2O2 | RAW 264.7 | [34,54,55,56,57,58,87] |
| HD-11EM | [101] | |
| hBMM/hPB | [103] | |
| mBMDM | [34,60,87] | |
| HL-60 | [100,102] | |
| U937 | [102] | |
| HP100-1 | [102] | |
| THP-1 | [100] | |
| hMSC | [104] | |
| mtROS | RAW 264.7 | [54,55,56,57] |
| hMSC/hPB | [15,56] | |
| NOX1 | mBMM/hBMM | [34,58,59,60,83,84] |
| RAW 264.7 | [54,58,91] | |
| NOX2 | mBMM | [59,60,62,83,84,105] |
| RAW 264.7 | [62,80,100] | |
| NOX4 | mBMM | [49,59,63,84] |
| RAW 264.7 | [63,64] |
| Compounds | Diseases | Basic Mechanisms/Treatment Indication | References |
|---|---|---|---|
| Alliin | Osteopenia | NOX/ROS inhibitor | [91] |
| Alpha-lipoic acid | Collagen-induced arthritis | Reduce ROS | [106] |
| Antioxidant enzymes | Osteoarthritis | NOX/ROS scavenger | [107] |
| Antioxidants (DPI, SOD, Peroxidase) | Osteoporosis, osteopenia, osteoarthritis | NOX/NOS/ROS inhibitor Flavoprotein inhibitor | [83] |
| Apocynin | Bone marrow senescence, osteoporosis | NOX/ROS scavenger | [83,108] |
| Epigallocatechin gallate | Orthodontic tooth movement | Reduce ROS | [92,93,94] |
| Extract of dried plum polyphenols | Osteoporosis | ROS and NFATc1 inhibitor NOX/ROS scavenger | [86] |
| EWHA-18278 | Osteoporosis/osteopenia | NOX/ROS inhibitor | [84] |
| Fermented oyster extract | Bone loss-related diseases | Reduce ROS | [109] |
| GKT136901, GKT137831 | Osteoporosis | NOX/ROS inhibitor | [83,89,90] |
| Lycopene | Postmenopausal bone loss | Reduce ROS | [97] |
| ML171/Thioridazine | NOX inhibitor | [85] | |
| Simvastatin | Rheumatoid arthritis | Reduce ROS, AKT, and MAPKs Promote bone formation | [87,88] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. https://doi.org/10.3390/ijms20143576
Agidigbi TS, Kim C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. International Journal of Molecular Sciences. 2019; 20(14):3576. https://doi.org/10.3390/ijms20143576
Chicago/Turabian StyleAgidigbi, Taiwo Samuel, and Chaekyun Kim. 2019. "Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases" International Journal of Molecular Sciences 20, no. 14: 3576. https://doi.org/10.3390/ijms20143576