Triphenylamine-Merocyanine-Based D1-A1-π-A2/A3-D2 Chromophore System: Synthesis, Optoelectronic, and Theoretical Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design
2.2. Synthesis and Characterization
2.3. Theoretical Calculations
2.4. Optical and Emission Properties
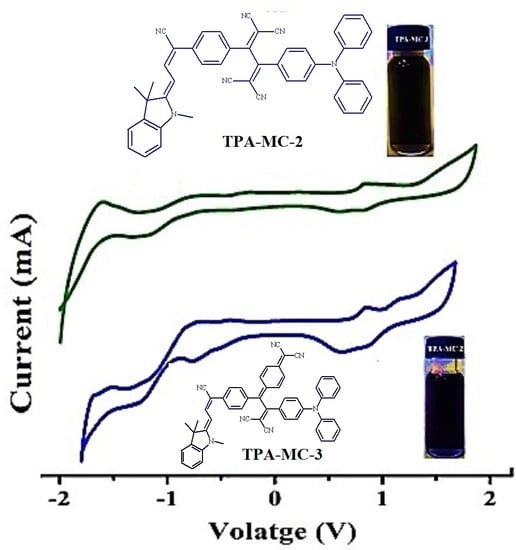
2.5. Electrochemical Properties
2.6. Discussion
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Synthetic Procedure of Compound 1
4.3. Synthetic Procedure of Compound 2
4.4. Synthetic Procedure of Compound 6
4.5. Synthetic Procedure of TPA-MC-1
4.6. Synthetic Procedure of TPA-MC-2
4.7. Synthetic Procedure of TPA-MC-3
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Supporting Information
Conflicts of Interest
Abbreviations
| CV | Cyclic Voltammetry |
| DCQDCM | Dicyanoquinodicyanomethane |
| HOMO | Highest occupied molecular orbital |
| ICT | Intramolecular charge-transfer |
| LUMO | Lowest unoccupied molecular orbital |
| MC | Merocyanine |
| TCBD | 1,1,4,4-tetracyanobutadiene |
| TCNE | Tetracyanoethene |
| TCNQ | 7.7.8,8-tetracyanoquinodimethane |
| TPA | Triphenylamine |
| UV–Vis | Ultraviolet Visible |
References
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Halls, J.J.M.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient photodiodes from interpenetrating polymer networks. Nature 1995, 376, 498–500. [Google Scholar] [CrossRef]
- Dempsey, J.L.; Esswein, A.J.; Manke, D.R.; Risenthal, J.; Soper, J.D.; Nocera, D.G. Molecular chemistry of consequence to renewable energy. Inorg. Chem. 2005, 44, 6879–6892. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Singh, S.P.; Johari, P. Strategical designing of donor–acceptor–donor based organic molecules for tuning their linear optical properties. J. Phys. Chem. A 2018, 122, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, D.; Ma, X.; Ansari, R.; Kim, J.; Kieffer, J. Design principles for the energy level tuning in donor/acceptor conjugated polymers. Phys. Chem. Chem. Phys. 2019, 21, 789–799. [Google Scholar] [CrossRef]
- Cnops, K.; Zango, G.; Genoe, J.; Heremans, P.; Martinez-Diaz, M.V.; Torres, T.; Cheyns, D. Energy level tuning of non-fullerene acceptors in organic solar cells. J. Am. Chem. Soc. 2015, 137, 8991–8997. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, M.; Kathiravan, A.; Solomon, R.V.; Jaccob, M. The role of π-linkers in tuning optoelectronic properties of triphenylamine derivatives for solar cell applications—A DFT/TDDFT study. Phys. Chem. Chem. Phys. 2017, 19, 6153–6163. [Google Scholar] [CrossRef]
- Tarsang, R.; Promarak, V.; Sudyoadsuk, T.; Namuangruk, S.; Jungsuttiwong, S. Tuning the electron donating ability in the triphenylamine-based D-π-A architecture for highly efficient dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2014, 273, 8–16. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, M.; Zhang, Q.; Zhang, Z.; Zhou, H.; Zhnag, S.; Li, S.; Wu, J.; Tian, Y. A series of triphenylamine-based two-photon absorbing materials with AIE property for biological imaging. J. Mater. Chem. B 2014, 2, 5430–5440. [Google Scholar] [CrossRef]
- Rao, P.S.; Gupta, A.; Zhang, F.; Bilic, A.; Xiang, W.; Evans, R.A.; Bhosale, S.V. Donor–acceptor–acceptor sketched non-fullerene acceptors carrying terminal chromen-2-one functionality for solution-processable organic photovoltaic devices. Dyes Pigm. 2017, 146, 502–511. [Google Scholar]
- Michinobu, T.; Diederich, F. The [2+2] cycloaddition-retrocyclization (CA-RE) click reaction: Facile access to molecular and polymeric push-pull chromophores. Angew. Chem. Int. Ed. 2018, 57, 2–28. [Google Scholar] [CrossRef] [PubMed]
- Kivala, M.; Diederich, F. Acetylene-derived strong organic acceptors for planar and non-planar push-pull chromophores. Acc. Chem. Res. 2009, 42, 235–248. [Google Scholar] [CrossRef]
- Zucchero, A.J.; McGrier, P.L.; Bunz, U.H.F. Cross-conjugated cruciform fluorophores. Acc. Chem. Res. 2010, 43, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Tykwinski, R.R. Oligomeric and polymeric systems with a cross-conjugated π-framework. Chem. Rev. 2006, 106, 4997–5027. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.C.W.; Hartley, C.S. A push–pull macrocycle with both linearly conjugated and cross-conjugated bridges. Org. Lett. 2013, 15, 3762–3765. [Google Scholar]
- Shoji, T.; Kamata, N.; Maruyama, A.; Ito, S.; Okujima, T. Synthesis, properties, and redox behavior of ferrocenyl-1,1,4,4-tetracyano-1,3-butadienes connected by arylamine and azobenzene spacers. Bull. Chem. Soc. Jpn. 2015, 88, 1338–1346. [Google Scholar] [CrossRef]
- Štefko, M.; Tzirakis, M.D.; Breiten, B.; Ebert, M.-O.; Dumele, O.; Schweiser, W.B.; Gisselbrecht, J.-P.; Boudon, C.; Beels, M.T.; Biaggio, I.; et al. Donor-acceptopr (D-A)-substituted polyyne chromophores: Modulation of their optoelectronic properties by varying the length of the acetylene spacers. Chem. Eur. J. 2013, 19, 12693–12704. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Liang, P.; Yang, Z.; Wang, D.; Cao, H.; He, W.; Yang, H.; Yu, L. Application of near-IR absorption porphyrin dyes derived from clock chemistry as third-order nonlinear optical materials. ChemistryOpen 2015, 5, 71–77. [Google Scholar] [CrossRef]
- Fujita, H.; Michinobu, T.; Fukuta, S.; Koganezawa, T.; Higashihara, T. Sequentially different AB diblock and ABA triblock copolymers as P3HT:PCBM interfacial compatibilizers for bulk-heterojunction photovoltaics. ACS Appl. Mater. Interfaces 2016, 8, 5484–5492. [Google Scholar] [CrossRef] [PubMed]
- Rout, Y.; Gautam, P.; Misra, R. Unsymmetrical and symmetrical push-pull phenothiazines. J. Org. Chem. 2017, 82, 6840–6845. [Google Scholar] [CrossRef]
- Rijkers, D.T.S.; Diederich, F. A convenient synthesis of new chromophoric tetracyanobutadiene-scaffolded peptides via a dipolar [2+2] cycloaddition-cycloreversion reactions. Tet. Lett. 2011, 52, 4021–4025. [Google Scholar] [CrossRef]
- Patil, Y.; Misra, R. Diketopyrrolopyrrole-based and tetracyano-bridged small molecules for bulk heterojunction organic solar cells. Chem. Asian J. 2018, 13, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Leliège, A.; Blanchard, P.; Rousseau, T.; Roncali, J. Triphenylamine/tetracyanobutadiene-based D-A-D π-conjugated systems as molecular donors for organic solar cells. Org. Lett. 2011, 13, 3098–3101. [Google Scholar] [CrossRef]
- Michinobu, T.; Satoh, N.; Cai, J.; Li, Y.; Han, L. Novel design of organic donor-acceptor dyes without carboxylic acid anchoring groups for dye-sensitized solar cells. J. Mater. Chem. C 2014, 2, 3367–3372. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, X.; Xue, X.; Li, Y.; Yu, A.; Chen, Y. Selective tuning of the HOMO–LUMO gap of carbazole-based donor–acceptor–donor compounds toward different emission colors. Eur. J. Org. Chem. 2010, 2010, 1681–1687. [Google Scholar] [CrossRef]
- Gregory, P. High Technology Application of Organic Colorants; Plenum: New York, NY, USA, 1991. [Google Scholar]
- Blanchard-Desce, M. Molecular engineering of NLO-phores for new NLO microscopies Nouvelles techniques d’imagerie multiphonique: Versune nouvelle génération de marqueurs moléculaires. C. R. Phys. 2002, 3, 439–448. [Google Scholar] [CrossRef]
- Verbiest, T.; Houbrechts, S.; Kauranen, M.; Clays, K.; Persoons, A. Second-order nonlinear optical materials: Recent advances in chromophore design. J. Mater. Chem. 1997, 7, 2175–2189. [Google Scholar] [CrossRef]
- Marder, S.R.; Perry, J.W. Molecular materials for second-order nonlinear optical applications. Adv. Mater. 1993, 5, 804–815. [Google Scholar] [CrossRef]
- Chen, R.; Yang, X.; Tian, H.; Wang, X.; Hagfeldt, A.; Sun, L. Effect of tetrahydroquinoline dyes structure on the performance of organic dye-sensitized solar cells. Chem. Mater. 2007, 19, 4007–4015. [Google Scholar] [CrossRef]
- Hagberg, D.P.; Edvinsson, T.; Mariando, T.; Boschloo, G.; Hagfeldt, A.; Sun, L. A novel organic chromophore for dye-sensitized nanostructured solar cells. Chem. Commun. 2006, 2245–2247. [Google Scholar] [CrossRef]
- Horiuchi, T.; Miura, H.; Uchida, S. Highly-efficient metal-free organic dyes for dye-sensitized solar cells. Chem. Commun. 2003, 3036–3037. [Google Scholar] [CrossRef]
- Li, S.; Jiang, K.; Shao, K.; Yang, L. Novel organic dyes for efficient dye-sensitized solar cells. Chem. Commun. 2006, 2792–2794. [Google Scholar] [CrossRef]
- Ito, S.; Zakeeruddin, S.M.; Humphry-Baker, R.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Péchy, P.; Takata, M.; Miura, H.; et al. High-efficiency organic-dye-sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv. Mater. 2006, 18, 1202–1205. [Google Scholar] [CrossRef]
- Sayama, K.; Hara, K.; Mori, N.; Satsuki, M.; Suga, S.; Tsukagoshi, S.; Abe, Y.; Sugihara, H.; Arakawa, H. Photosensitization of a porous TiO2 electrode with merocyanine dyes containing a carboxyl group and a long alkyl chain. Chem. Commun. 2000, 1173–1174. [Google Scholar] [CrossRef]
- Würthner, F.; Thalacker, C.; Matschiner, R.; Lukaszuk, K.; Wortmann, R. Optimization of neutrocyanine chromophores based on five-membered heterocycles for photorefractive applications. Chem. Commun. 1998, 1739–1740. [Google Scholar] [CrossRef]
- Beckmann, S.; Etzbach, K.-H.; Krämer, P.; Lukaszuk, K.; Matschiner, R.; Schmidt, A.J.; Schuhmacher, P.; Sens, R.; Seybold, G.; Wortmann, R.; et al. Electrooptical chromophores for nonlinear optical and photorefractive applications. Adv. Mater. 1999, 11, 536–541. [Google Scholar] [CrossRef]
- Würthner, F.; Yao, S.; Schilling, J.; Wortmann, R.; Redi-Abshiro, M.; Mecher, E.; Gallego-Gomez, F.; Meerholz, K. ATOP dyes: Optimization of a multifunctional merocyanine chromophore for high refractive index modulation in photorefractive materials. J. Am. Chem. Soc. 2001, 123, 2810–2824. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, N.M.; Deppisch, M.; Würthner, F.; Lademann, H.W.A.; Deing, K.; Meerholz, K. Bulk heterojunction organic solar cells based on merocyanine colorants. Chem. Commun. 2008, 6489–6491. [Google Scholar] [CrossRef] [PubMed]
- Gräßler, N.; Wolf, S.; Holzmüller, F.; Zeika, O.; Vandewal, K.; Leo, K. Heteroquinoid merocyanine dyes with high thermal stability as absorber materials in vacuum-processed organic solar cells. Eur. J. Org. Chem. 2019, 31, 845–851. [Google Scholar] [CrossRef]
- Bürckstümmer, H.; Kronenberg, N.M.; Gsänger, M.; Stolte, M.; Meerholz, K.; Würthner, F. Tailored merocyanine dyes for solution-processed BHJ solar cells. J. Mater. Chem. 2010, 20, 240–243. [Google Scholar] [CrossRef]
- Arjona-Esteban, A.; Lenze, M.R.; Meerholz, K.; Würthner, F. Donor-acceptor dyes for organic photovoltaics. In Elementary Processes in Organic Photovoltaics; Leo, K., Ed.; Springer International Publisher: Cham, Switzerland, 2017; pp. 193–214. [Google Scholar]
- Ojala, A.; Petersen, A.; Fuchs, A.; Lovrincic, R.; Pölking, C.; Trollmann, J.; Hwang, J.; Lennartz, C.; Reichelt, H.; Höffken, H.W.; et al. Merocyanine/C60 planar heterojucntion solar cells: Effect of dye orientation on exciton dissociation and solar cell performance. Adv. Funct. Mater. 2012, 22, 86–96. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Stylianakis, M.M.; Mikroyannidis, J.A.; Dong, Q.; Pei, J.; Tian, W. Synthesis, photophysical and photovoltaic properties of star-shaped molecules with triphenylamine as core and phenylethenylthiophene or dithienylethylene as arms. Sol. Energy Mater. Sol. Cells 2009, 93, 1952–1958. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhou, Y.; Zhao, G.; He, C.; Li, Y.; Andersson, M.; Inganäs, O.; Zhang, F. Solution-processable organic molecule with triphenylamine core and two benzothiadiazole-thiophene arms for photovoltaic application. J. Phys. Chem. C 2010, 114, 3701–3706. [Google Scholar] [CrossRef]
- Yasuda, T.; Shinohara, Y.; Matsuda, T.; Han, L.; Ishi-I, T. Improved power conversion efficiency of bulk-heterojunction organic solar cells using a benzothiadiazole–triphenylamine polymer. J. Mater. Chem. 2012, 22, 2539–2544. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Stylianakis, M.M.; Suresh, P.; Balraju, P.; Sharma, G.D. Low band gap vinylene compounds with triphenylamine and benzothiadiazole segments for use in photovoltaic cells. Org. Electron. 2009, 10, 1320–1333. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, B.; Zheng, X.; Zhao, Z.; Deng, P.; Lin, M.; Tang, B.; Ong, B.S. Novel dimethylmethylene-bridged triphenylamine-PDI acceptor for bulk-heterojunction organic solar cells. Adv. Sci. 2017, 4, 1700110. [Google Scholar] [CrossRef]
- Ouyang, M.; Wang, G.; Zhang, C. A novel electrochromic polymer containing triphenylamine derivative and pyrrole. Electrochim. Acta 2011, 56, 4645–4649. [Google Scholar] [CrossRef]
- Yao, S.; Belfield, K.D. Two-photon fluorescent probes for bioimaging. Eur. J. Org. Chem. 2012, 2012, 3199–3217. [Google Scholar] [CrossRef]
- Shirota, Y. Organic materials for electronic and optoelectronic devices. J. Mater. Chem. 2000, 10, 1–25. [Google Scholar] [CrossRef]
- Roncali, J. Molecular bulk heterojunctions: An emerging approach to organic solar cells. Acc. Chem. Res. 2009, 42, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- He, C.; He, Q.; Yang, X.; Wu, G.; Yang, C.; Bai, F.; Shuai, Z.; Wang, L.; Li, Y. Synthesis and photovoltaic properties of a solution-processable organic molecule containing triphenylamine and DCM moieties. J. Phys. Chem. C 2007, 111, 8661–8666. [Google Scholar] [CrossRef]
- Srivani, D.; Gupta, A.; Bhosale, S.V.; Ohkubo, K.; Bhosale, R.S.; Fukuzumi, S.; Bilic, A.; Jones, L.A.; Bhosale, S.V. A Triphenylamine–naphthalenediimide–fullerene triad: Synthesis, photoinduced charge separation and solution-processable bulk heterojunction solar cells. Asian J. Org. Chem. 2018, 7, 220–226. [Google Scholar] [CrossRef]
- Bruce, M.I.; Rodgers, J.R.; Snow, M.R.; Swincer, A.G. Cyclopentadienyl-ruthenium and -osmium chemistry. cleavage of tetracyanoethylene under mild conditions: X-ray crystal structures of [Ru{η3-C(CN)2CPhC=C(CN)2}(PPh3)(η-C5H5)] and [Ru{C[=C(CN)2]CPh=C(CN)2}-(CNBut)(PPh3)(η-C5H5)]. J. Chem. Soc. Chem. Commun. 1981, 271–272. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; You, W. Rational design of high performance conjugated polymers for organic solar cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Coughlin, J.E.; Henson, Z.B.; Welch, G.C.; Bazan, G.C. Design and synthesis of molecular donors for solution-processed high-efficiency organic solar cells. Acc. Chem. Res. 2014, 47, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Q.; Li, Z.; Pei, J.; Tian, W. Solution processable D–A small molecules for bulk-heterojunction solar cells. Energy Environ. Sci. 2010, 3, 1427–1436. [Google Scholar] [CrossRef]
- Gao, H.; Li, Y.; Wang, L.; Ji, C.; Wang, Y.; Tian, W.; Yang, X.; Yin, L. High performance asymmetrical push–pull small molecules end-capped with cyanophenyl for solution-processed solar cells. Chem. Commun. 2014, 50, 10251–10254. [Google Scholar] [CrossRef] [PubMed]
- Cabau, L.; Pellejà, L.; Clifford, J.N.; Kumar, C.V.; Palomares, E. Light soaking effects on charge recombination and device performance in dye sensitized solar cells based on indoline–cyclopentadithiophene chromophores. J. Mater. Chem. A 2013, 1, 8994–9000. [Google Scholar] [CrossRef]
- Ma, X.; Azeem, E.A.; Liu, X.; Cheng, Y.; Zhu, C. Synthesis and tunable chiroptical properties of chiral BODIPY-based D–π–A conjugated polymers. J. Mater. Chem. C 2014, 2, 1076–1084. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Song, Y.; Xu, W.; Zhang, D.; Zhu, D. Novel diethynylcarbazole macrocycles: synthesis and optoelectronic properties. J. Org. Chem. 2006, 71, 7422–7432. [Google Scholar] [CrossRef] [PubMed]
- Suraru, S.-L.; Würthner, F. Regioselectivity in sequential nucleophilic substitution of tetrabromonaphthalene diimides. J. Org. Chem. 2013, 78, 5227–5238. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool, Version 1.1.0. Available online: http://avogadro.openmolecules.net/ (accessed on 31 March 2019).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]







| Acceptor | λmax Solution (nm) | λmax Film (nm) | λonset Value Film (nm) | Optical Bandgap Eg (eV) a |
|---|---|---|---|---|
| TPA-MC-1 | 442, 364, 313 | 445, 315 | 776 | 1.59 |
| TPA-MC-2 | 595, 481, 282 (Shoulder) | 631, 498, 300 | 986 | 1.25 |
| TPA-MC-3 | 663, 483, 291 | 670, 485, 300 | 1083 | 1.14 |
| Acceptor | Eoxonset (V) | Eredonset (V) | HOMO = [Eredonset + 4.8] (eV) | LUMO = [Eredonset + 4.8] (eV) | Eg = (HOMO-LUMO) eV |
|---|---|---|---|---|---|
| TPA-MC-1 | 0.81 | −0.89 | −5.21 | −3.47 | 1.74 |
| TPA-MC-2 | 0.82 | −0.39 | −5.22 | −4.01 | 1.21 |
| TPA-MC-3 | 0.79 | −0.24 | −5.19 | −4.16 | 1.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasa Rao, P.; L. Puyad, A.; V. Bhosale, S.; V. Bhosale, S. Triphenylamine-Merocyanine-Based D1-A1-π-A2/A3-D2 Chromophore System: Synthesis, Optoelectronic, and Theoretical Studies. Int. J. Mol. Sci. 2019, 20, 1621. https://doi.org/10.3390/ijms20071621
Srinivasa Rao P, L. Puyad A, V. Bhosale S, V. Bhosale S. Triphenylamine-Merocyanine-Based D1-A1-π-A2/A3-D2 Chromophore System: Synthesis, Optoelectronic, and Theoretical Studies. International Journal of Molecular Sciences. 2019; 20(7):1621. https://doi.org/10.3390/ijms20071621
Chicago/Turabian StyleSrinivasa Rao, Pedada, Avinash L. Puyad, Sidhanath V. Bhosale, and Sheshanath V. Bhosale. 2019. "Triphenylamine-Merocyanine-Based D1-A1-π-A2/A3-D2 Chromophore System: Synthesis, Optoelectronic, and Theoretical Studies" International Journal of Molecular Sciences 20, no. 7: 1621. https://doi.org/10.3390/ijms20071621