Resilience of Soil Microbial Communities to Metals and Additional Stressors: DNA-Based Approaches for Assessing “Stress-on-Stress” Responses
Abstract
:1. Introduction
2. Stability, Resilience and Tolerance of Microbial Communities
3. Response of Microbial Communities to Metal–Pollution Stress
4. Mechanisms of Metal Adaptation and Tolerance in Microbial Communities
5. Stability of Microbial Communities (Resistance and Resilience) to Secondary Stressors in Metal-Polluted Environments
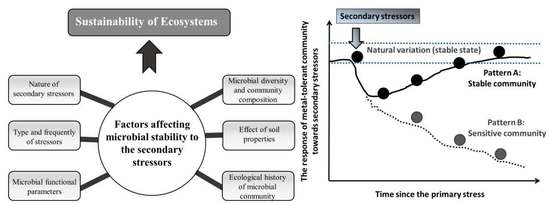
6. Factors Affecting Microbial Responses to Secondary Stressors
6.1. Nature of Secondary Stressors
6.2. Knowledge of Diversity, Composition and Function of Microbial Communities before and after Secondary Stressors
6.3. Microbial Functional Parameters
6.4. Field vs. Laboratory Experiments
6.5. Effect of Soil Properties
7. Conclusions
- (1)
- The application of new DNA-based methodologies, allowing culture-independent assessment of microbial communities, has greatly enhanced our insights into the composition of these communities and their functional capacities.
- (2)
- There is increasing support that the historical environmental conditions and disturbance regimes are primary factors to influence microbial community stability towards secondary stressors. We argue that knowledge of factors controlling the composition and function of microbial communities is pivotal when attempting to predict the impact of secondary stressors on the stability of soil microbial communities. The potential influence of past events (such as metal pollution) on the development of community co-tolerance highlights the importance of historical factors in predicting the response of microbial communities to additional stressors.
- (3)
- Long-term “stress-on-stress” studies with single metals or defined mixtures may provide an integrated insight into the complex responses of soil microbial communities to secondary stressors at polluted sites. Following severe or long-term stress exposure, microbial community may not be able to recover within a short-term period.
- (4)
- The effect of soil physicochemical properties is important. The effects of metal pollution on microbial community structure is often confounded by natural soil properties such as pH and organic matter content.
- (5)
- Detailed knowledge on the functional and compositional properties of the microbial community prior to applying additional stressors may help to evaluate and interpret the microbial response to these stressors.
- (6)
- In contrast to DGGE and PLFA, far less attention has been given to sequence data to examine the direct or indirect interactions between microbial taxa in the face of stressors. We recommend that future “stress-on-stress” studies should investigate microbial communities under environmentally stressful conditions, using metagenomics and meta-transcriptomics together with carefully designed disturbance experiments and using different types of stressors. We expect that such studies will provide a deeper insight and clearer picture on whether predictable species assemblages occur following the combination of metal stress with other environmental stressors.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Straalen, N.M. Assessment of soil contamination—A functional perspective. Biodegradation 2002, 13, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Quince, C.; Macdonald, C.A.; Khachane, A.; Thomas, N.; AlSoud, W.A.; Sørensen, S.J.; He, Z.; White, D.; Sinclair, A.; et al. Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 2014, 16, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; McClaugherty, C.; Virzo de Santo, A.; Johansson, M.B.; Ekbohm, G. Decomposition of litter and soil organic matter—Can be distinguish a mechanism for soil organic matter buildup? Scand. J. For. Res. 1995, 10, 108–119. [Google Scholar] [CrossRef]
- Vesterdal, L.; Dalsgaard, M.; Felby, C.; Raulund-Rasmussen, K.; Jørgensen, B. Effects of thinning and soil properties on accumulation of carbon, nitrogen and phosphorus in the forest floor of Norway spruce stands. For. Ecol. Manag. 1995, 77, 1–10. [Google Scholar] [CrossRef]
- Nannipieri, P.; Grego, S.; Ceccanti, B. Ecological significance of the biological activity in soil. In Soil Biochemistry; Bollag, J.-M., Stotzky, G., Eds.; Marcel Dekker: New York, NY, USA, 1990; Volume 6, pp. 293–355. [Google Scholar]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Berg, B.; Ekbohm, G.; Söderström, B.; Staaf, H. Reduction of decomposition rates of Scots pine needle litter due to heavy-metal pollution. Water Air Soil Pollut. 1991, 69, 165–177. [Google Scholar] [CrossRef]
- Van Gestel, C.A.M. Soil ecotoxicology: State of the art and future directions. ZooKeys 2012, 176, 275–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardestani, M.M.; van Straalen, N.M.; van Gestel, C.A.M. Uptake and elimination kinetics of metals in soil invertebrates: A review. Environ. Pollut. 2014, 193, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Stotzky, G. Heavy metal toxicity to microbe-mediated ecologic processes: A review and potential application to regulator policies. Environ. Res. 1985, 36, 111–137. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S.P. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Botton, S.; van Heusden, M.; Parsons, J.R.; Smidt, H.; van Straalen, N. Resilience of microbial systems towards disturbances. Crit. Rev. Microbiol. 2006, 32, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2012, 37, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Deng, H. A review of diversity-stability relationship of soil microbial community: What do we not know? J. Environ. Sci. 2012, 24, 1027–1035. [Google Scholar] [CrossRef]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.; Berga, M.; Buergmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.; et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Smolders, E. Background zinc concentrations in soil affect the zinc sensitivity of soil microbial processes—A rationale for a metalloregion approach to risk assessments. Environ. Toxicol. Chem. 2001, 20, 2639–2643. [Google Scholar] [PubMed]
- Azarbad, H.; Niklińska, M.; Nikiel, K.; van Straalen, N.M.; Röling, W.F.M. Functional and compositional responses in soil microbial communities along two metal pollution gradients: Does the level of historical pollution affect resistance against secondary stress? Biol. Fertil. Soils 2015, 51, 879–890. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Kunito, T.; Senoo, K.; Saeki, K.; Oyaizu, H.; Matsumoto, S. Usefulness of the sensitivity-resistance index to estimate the toxicity of copper on bacteria in copper-contaminated soils. Ecotoxicol. Environ. Saf. 1999, 44, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Azarbad, H.; Niklińska, M.; van Gestel, C.A.M.; van Straalen, N.M.; Röling, W.F.M.; Laskowski, R. Microbial community structure and functioning along metal pollution gradients. Environ. Toxicol. Chem. 2013, 32, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Ryan, D.J.; Dowling, D.N. Multiple metal resistant transferable phenotypes in bacteria as indicators of soil contamination with heavy metals. J. Soil Sed. 2005, 5, 95–100. [Google Scholar] [CrossRef]
- Cai, L.; Liu, G.; Rensing, C.; Wang, G. Genes involved in arsenic transformation and resistance associated with different levels of arsenic contaminated soils. BMC Microbiol. 2009, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Summers, A.O.; Wireman, J.; Vimy, M.J.; Lorscheider, F.L.; Marshall, B.; Levy, S.B.; Bennett, S.; Billard, L. Mercury released from dental “silver” fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob. Agents Chemother. 1993, 37, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Singh, A.; Ramteke, P.W.; Singh, V.P. Characterization of large plasmids encoding resistance to toxic heavy metals in Salmonella abortus equi. Biochem. Biophys Res. Commun. 2000, 272, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.H.; Thornton, I. The resistance patterns to metals of bacterial population in contaminated land. J. Soil Sci. 1982, 33, 271–277. [Google Scholar] [CrossRef]
- Hemme, C.L.; Deng, Y.; Gentry, T.J.; Fields, M.W.; Wu, L.; Barua, S.; Barry, K.; Tringe, S.G.; Watson, D.B.; He, Z.; et al. Metagenomic insights into evolution of a heavy metal-contaminated groundwater microbial community. ISME J. 2010, 4, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; van Nostrand, J.D.; Gough, H.L.; He, Z.; Hazen, T.C.; Stahl, D.A.; Zhou, J. Functional gene array-based analysis of microbial communities in heavy metals-contaminated lake sediments. FEMS Microbiol. Ecol. 2013, 86, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, F.; Maelfait, J.P.; Lens, L. Effect of metal stress on life history divergence and quantitative genetic architecture in a wolf spider. J. Evol. Biol. 2008, 21, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Van Straalen, N.M.; Burghouts, T.B.A.; Doornhof, M.J.; Groot, G.M.; Jansens, M.P.M.; Joose, E.N.G.; van Meerendonk, J.H.; Theeuwen, J.P.J.J.; Verhoef, H.A.; Zoomer, H.R. Efficiency on lead and cadmium excretion in populations of Orchesella cincta (Collembola) from variuos contaminatrd forest soils. J. Appl. Ecol. 1987, 24, 953–968. [Google Scholar] [CrossRef]
- Mertens, J.; Wakelin, S.A.; Broos, K.; McLaughlin, M.J.; Smolders, E. Extent of copper tolerance and consequences for functional stability of the ammonia oxidizing community in long-term copper-contaminated soils. Environ. Toxicol. Chem. 2010, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Ruyters, S.; Springael, D.; Smolders, E. Resistance and resilience of zinc tolerant nitrifying communities is unaffected in long term zinc contaminated soil. Soil Biol. Biochem. 2007, 39, 1828–1831. [Google Scholar] [CrossRef]
- Tobor-Kapłon, MA.; Bloem, J.; Römkens, P.F.A.M.; de Ruiter, P.C. Functional stability of microbial communities in contaminated soils near a zinc smelter (Budel, The Netherlands). Ecotoxicology 2006, 15, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Cregut, M.; Chèneby, D.; Bressan, M.; Dequiet, S.; Martin-Laurent, F.; Ranjard, L.; Lemanceau, P. Effect of primary mild stresses on resilience and resistance of the nitrate reducer community to a subsequent severe stress. FEMS Microbiol. Lett. 2008, 285, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tobor-Kapłon, M.A.; Bloem, J.; Römkens, P.F.A.M.; de Ruiter, P.C. Functional stability of microbial communities in contaminated soils. Oikos 2005, 111, 119–129. [Google Scholar] [CrossRef]
- Müller, A.K.; Rasmussen, L.D.; Sørensen, S.J. Adaptation of the bacterial community to mercury contamination. FEMS Microbiol. Lett. 2001, 204, 49–53. [Google Scholar] [CrossRef]
- Kools, S.A.E.; Berg, M.P.; Boivin, M.E.Y.; Kuenen, F.J.A.; van der Wurff, A.G.; van Gestel, C.A.M.; van Straalen, N.M. Stress responses investigated; application of zinc and heat to Terrestrial Model Ecosystems from heavy metal polluted grassland. Sci. Total Environ. 2008, 406, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982; p. 296. [Google Scholar]
- McNaughton, S.J. Biodiversity and stability of grazing ecosystems. In Biodiversity and Ecosystem Function; Shulze, E.D., Mooney, H.A., Eds.; Springer-Verlag: Berlin, Germany, 1994; pp. 361–383. [Google Scholar]
- Zhang, B.; Wang, H.; Yao, S.; Bi, L. Litter quantity confers soil functional resilience through mediating soil biophysical habitat and microbial community structure on an eroded bare land restored with mono Pinus massoniana. Soil Biol. Biochem. 2013, 57, 556–567. [Google Scholar] [CrossRef]
- Blanck, H.; Wängberg, S.A.; Molander, S. Pollution-induced community tolerance—A new ecotoxicological tool. In Functional Testing of Aquatic Biota for Estimating Hazards of Chemicals; Cairns, J., Pratt, J.R., Eds.; American Society for Testing and Materials: Philadelphia, PA, USA, 1988; pp. 219–230. [Google Scholar]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Pereira e Silva, M.; Semenov, A.V.; Schmitt, H.; van Elsas, J.D.; Salles, J.F. Microbemediated processes as indicators to establish the normal operating range of soil functioning. Soil Biol. Biochem. 2013, 57, 955–1002. [Google Scholar] [CrossRef]
- Brandt, K.K.; Amézquita, A.; Backhaus, T.; Boxall, A.; Coors, A.; Heberer, T.; Lawrence, J.R.; Lazorchak, J.; Schönfeld, J.; Snape, J.R.; et al. Ecotoxicological assessment of antibiotics: A call for improved consideration of microorganisms. Environ. Int. 2015, 85, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Chaudri, A.M.; McGrath, S.P.; Gibbs, P.; Chambers, B.C.; Carlton-Smith, C.; Bacon, J.; Campbell, C.; Aitken, A. Population size of indigenous Rhizobium leguminosarum biovar trifolii in long-term field experiments with sewage sludge cake, metal-amended liquid sludge or metal salts: Effects of zinc, copper and cadmium. Soil Biol. Biochem. 2008, 40, 1670–1680. [Google Scholar] [CrossRef]
- Mertens, J.; Springael, D.; de Troyer, I.; Cheyns, K.; Wattiau, P.; Smolders, E. Long-term exposure to elevated zinc concentrations induced structural changes and zinc tolerance of the nitrifying community in soil. Environ. Microbiol. 2006, 8, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Berdicevsky, I.; Duek, L.; Merzbach, D.; Yannai, S. Susceptibility of different yeast species to environmental toxic metals. Environ. Pollut. 1993, 80, 41–44. [Google Scholar] [CrossRef]
- Romandini, P.; Tallandini, L.; Beltramini, M.; Salvato, B.; Manzano, M. Effects of copper and cadmium on growth, superoxide dismutase and catalase activities in different yeast strains. Comp. Biochem. Physiol. 1992, 103C, 255–262. [Google Scholar]
- Oliveira, A.; Pampulha, M.E.; Neto, M.M.; Almeida, A.C. Mercury tolerant diazotrophic bacteria in a long-term contaminated soil. Geoderma 2010, 154, 359–363. [Google Scholar] [CrossRef]
- Sheik, C.S.; Mitchell, T.W.; Rizvi, F.Z.; Rehman, Y.; Faisal, M.; Hasnain, H.; McInerney, M.J.; Krumholz, L.R. Exposure of soil microbial communities to chromium and arsenic alters their diversity and structure. PLoS ONE 2012, 7, e40059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, Z.; Liang, Z.; Stoffella, P.J.; Fan, J.; Yang, Y.; Powell, C.A. Long-term use of copper-containing fungicide affects microbial properties of citrus grove soils. Soil Sci. Am. J. 2011, 75, 898–906. [Google Scholar] [CrossRef]
- Joynt, J.; Bischoff, M.; Turco, R.; Konopka, A.; Nakatsu, C.H. Microbial community analysis of soils contaminaed with lead, chromium and petroleum hydrocarbons. Microb. Ecol. 2006, 51, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; He, Q.; Hemme, C.; Mukhopadhyay, A.; Hillesland, K.; Zhou, A.; He, Z.; van Nostrand, J.D.; Hazen, T.C.; Stahl, D.A.; et al. How sulphate-reducing microorganisms cope with stress: Lessons from systems biology. Nat. Rev. Microbiol. 2011, 9, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.J.; Yeakel, K.L.; Bates, N.R.; de Putron, S.J. Partial offsets in ocean acidification from changing coral reef biogeochemistry. Nat. Clim. Chang. 2014, 4, 56–61. [Google Scholar] [CrossRef]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Grimm, V.; Wissel, C. Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 1997, 109, 323–334. [Google Scholar] [CrossRef]
- Hodgson, D.; McDonald, J.L.; Hosken, D.J. What do you mean, “resilient”? Trends Ecol. Evol. 2015, 30, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2012, 49, 22–30. [Google Scholar] [CrossRef]
- Van Straalen, N.M.; Roelofs, D. An Introduction to Ecological Genomics; Oxford University Press: New York, NY, USA, 2006; p. 299. [Google Scholar]
- Penanen, T.; Frostegård, A.; Fritze, H.; Bååth, E. Phospholipid fatty acid composition and heavy metal tolerance of soil microbial communities along two heavy metal-polluted gradients in coniferous forests. Appl. Environ. Microbiol. 1996, 62, 420–428. [Google Scholar]
- Bååth, E.; Díaz-Ravina, M.; Frostegård, A.; Campbell, C.D. Effect of metal-rich sludge amendments on the soil microbial community. Appl. Environ. Microbiol. 1998, 64, 238–245. [Google Scholar]
- Bååth, E.; Frostegård, A.; Díaz-Ravina, M.; Tunlid, A. Microbial community based measurements to estimate heavy metal effects in soil: The use of phospholipid fatty acid patterns and bacterial community tolerance. AMBIO 1998, 27, 58–62. [Google Scholar]
- Kelly, J.J.; Haggblom, M.; Tate, R.L. Effects of the land application of sewage sludge on heavy metal concentrations and soil microbial communities. Soil Biol. Biochem. 1999, 31, 1467–1470. [Google Scholar] [CrossRef]
- Witter, E.; Gong, P.; Bååth, E. A study of the structure and metal tolerance of the soil microbial community six years after cessation of sewage sludge applications. Environ. Toxicol. Chem. 2000, 19, 1983–1991. [Google Scholar] [CrossRef]
- Harris-Hellal, J.; Vallaeys, T.; Garnier-Zarli, E.; Bousserrrhine, N. Effects of mercury on soil microbial communities in tropical soils of French Guyana. Appl. Soil Ecol. 2009, 41, 59–68. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; Bååth, E. Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl. Environ. Microbiol. 1996, 62, 2970–2972. [Google Scholar] [PubMed]
- Bååth, E.; Díaz-Raviña, M.; Bakken, L.R. Microbial biomass, community structure and metal tolerance of a naturally Pb-enriched forest soil. Microbiol. Ecol. 2005, 50, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Gołębiewski, M.; Deja-Sikora, E.; Cichosz, M.; Tretyn, A.; Wrobel, B. 16S rDNA pyrosequencing analysis of bacterial community in heavy metals polluted soils. Microb. Ecol. 2014, 67, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Brandt, K.K.; Al-Soud, W.A.; Holm, P.E.; Hansen, L.H.; Sorensen, S.J.; Nybroe, O. Selection for Cu-tolerant bacterial communities with altered composition, but unaltered richness, via long-term Cu exposure. Appl. Environ. Microbiol. 2012, 78, 7438–7446. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.K.; Frandsen, R.J.N.; Holm, P.E.; Nybroe, O. Development of pollution-induced community tolerance is linked to structural and functional resilience of a soil bacterial community following a five-year field exposure to copper. Soil Biol. Biochem. 2010, 49, 748–757. [Google Scholar] [CrossRef]
- Hong, C.; Si, Y.X.; Xing, Y.; Li, Y. Illumina MiSeq sequencing investigation on the contrasting soil bacterial community structures in different iron mining areas. Environ. Sci. Pollut. 2015, 22, 10788–10799. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, H.W.; Ma, Y.B.; Wang, J.T.; Liu, Y.R.; He, J.Z. Long-term nickel exposure altered the bacterial community composition but not diversity in two contrasting agricultural soils. Environ. Sci. Pollut. 2015, 2, 10496–10505. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Li, J.T.; Chen, Y.T.; Huang, L.N.; Hua, Z.S.; Hu, M.; Shu, W.S. Shifts in microbial community composition and function in the acidification of a lead/zinc mine tailings. Environ. Microbiol. 2013, 15, 2431–2444. [Google Scholar] [CrossRef] [PubMed]
- Chodak, M.; Gołębiewski, M.; Morawska-Płoskonka, J.; Kuduk, K.; Niklińska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- De Boer, TE.; Taş, N.; Braster, M.; Temminghoff, E.J.M.; Röling, W.F.M.; Roelofs, D. The influence of long-term copper contaminated agricultural soil at different pH levels on microbial communities and springtail transcriptional regulation. Environ. Sci. Technol. 2012, 46, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Renella, G.; Mench, M.; Landi, L.; Nannipieri, P. Microbial diversity and hydrolase synthesis in long-term Cd-contaminated soils. Soil Biol. Biochem. 2005, 37, 133–139. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Y.M.; Liu, Y.R.; Ma, Y.B.; Hu, H.W.; He, J.Z. Initial copper stress strengthens the resistance of soil microorganisms to a subsequent copper stress. Microb. Ecol. 2014, 67, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Bourceret, A.; Cébron, A.; Tisserant, E.; Poupin, P.; Bauda, P.; Beguiristain, T.; Leyval, C. The bacterial and fungal diversity of an aged PAH- and heavy metal contaminated soil is affected by plant cover and edaphic parameters. Microb. Ecol. 2015, 71, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Epelde, L.; Becerril, J.M.; Kowalchuk, G.A.; Deng, Y.; Zhou, J.-Z.; Garbisu, C. Impact of metal pollution and Thlaspi caerulescens growth on soil microbial communities. Appl. Environ. Microbiol. 2010, 76, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Azarbad, H.; Niklińska, M.; Laskowski, R.; van Straalen, N.M.; van Gestel, C.A.M.; Zhou, J.; He, Z.; Wen, C.; Röling, W.F.M. Microbial community composition and functions are resilient to metal pollution along two forest soil gradients. FEMS Microbiol. Ecol. 2015, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Bennett, J.; Fierer, N. Microbial community composition and soil nitrogen cycling: Is there really a connection? In Biological Diversity and Function in Soils; Bardgett, R.D., Hopkins, D.W., Usher, M.B., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 171–188. [Google Scholar]
- Martiny, A.C.; Treseder, K.; Pusch, G. Phylogenetic conservatism of functional traits in microorganisms. ISME 2013, 7, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Niklińska, M.; Chodak, M.; Laskowski, R. Pollution-induced community tolerance of microorganisms from forest soil organic layers polluted with Zn or Cu. Appl. Soil Ecol. 2006, 32, 265–272. [Google Scholar] [CrossRef]
- Chau, J.F.; Bagtzoglou, A.C.; Wilig, M.R. The effect of soil texture on richness and diversity of bacterial communities. Environ. Forensics 2011, 12, 333–341. [Google Scholar] [CrossRef]
- Carson, J.K.; Gonzalez-Quinones, V.; Murphy, D.V.; Hinz, C.; Shaw, J.A.; Gleeson, D.B. Low pore connectivity increases bacterial diversity in soil. Appl. Environ. Microbiol. 2010, 76, 3936–3942. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Niklińska, M.; Laskowski, R. Metals affect soil bacterial and fungal functional diversity differently. Environ. Toxicol. Chem. 2008, 27, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metals. Soil Biol. Biochem. 1996, 28, 55–63. [Google Scholar] [CrossRef]
- Turpeinen, R.; Kairesalo, T.; Häggblom, M.M. Microbial community structure and activity in arsenic, chromium and copper contaminated soils. FEMS Microbiol. Ecol. 2004, 47, 39–50. [Google Scholar] [CrossRef]
- Lejon, D.P.H.; Nowak, V.; Bouko, S.; Pascault, N.; Mougel, C.; Martins, J.M.; Ranjard, L. Fingerprinting and diversity of bacterial copA genes in response to soil types, soil organic status and copper contamination. FEMS Microbiol. Ecol. 2007, 61, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H.; Wängberg, S.A. Induced community tolerance in marine periphyton established under arsenate stress. Can. J. Fish. Aquat. Sci. 1988, 45, 1816–1819. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T. Bacterial heavy metal resistance: New surprises. Annu. Rev. Microbiol. 1996, 50, 753–789. [Google Scholar] [CrossRef] [PubMed]
- Jaroslawiecka, A.; Piotrowska-Seget, Z. Lead resistance in micro-organisms. Microbiology 2014, 160, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Klerks, P.L.; Weis, J.S. Genetic adaptation to heavy metals in aquatic organisms: A review. Environ. Pollut. 1987, 45, 173–205. [Google Scholar] [CrossRef]
- Puglisi, E.; Hamon, R.E.; Vasileiadis, S.; Coppolecchia, D.; Trevisan, M. Adaptation of soil microorganisms to trace element contamination: A review of mechanisms, methodologies and consequences for risk assessment and remediation. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2435–2470. [Google Scholar] [CrossRef]
- Blanck, H. A critical review of procedures and approaches used for assessing pollution-induced community tolerance (PICT) in biotic communities. Hum. Ecol. Risk Assess. 2002, 8, 1003–1034. [Google Scholar] [CrossRef]
- Almås, Å.R.; Bakken, L.R.; Mulder, J. Changes in tolerance of soil microbial communities in Zn and Cd contaminated soils. Soil Biol. Biochem. 2004, 36, 805–813. [Google Scholar] [CrossRef]
- Davis, M.R.H.; Zhao, F.; McGrath, S.P. Pollution-induced community tolerance of soil microbes in response to a zinc gradient. Environ. Toxicol. Chem. 2004, 23, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Raviña, M.; Bååth, E.; Frostegård, A. Multiple heavy metal tolerance of soil bacterial communities and its measurement by a thymidine incorporation technique. Appl. Environ. Microbiol. 1994, 60, 2238–2247. [Google Scholar] [PubMed]
- Vinebrooke, R.D.; Cottingham, K.L.; Norberg, J.; Scheffer, M.; Dodson, S.I.; Maberly, S.C.; Sommer, U. Impacts of multiple stressors on biodiversity and ecosystem functioning: The role of species co-tolerance. Oikos 2004, 104, 451–457. [Google Scholar] [CrossRef]
- Ruyters, S.; Mertens, J.; Springael, D.; Smolders, E. Stimulated activity of the soil nitrifying community accelerates community adaptation to Zn stress. Soil Biol. Biochem. 2010, 42, 766–772. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Li, X.F.; Cheng, W.D.; Zhu, Y.G. Resistance and resilience of Cu-polluted soil after Cu perturbation, tested by a wide range of soil microbial parameters. FEMS Microbiol. Ecol. 2009, 70, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.S. Disinfectant resistance mechanisms, cross resistance, and co-resistance. Int. Biodeterior. Biodegrad. 2003, 51, 271–276. [Google Scholar] [CrossRef]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef]
- Day, T. Competition and the effect of spatial resource heterogeneity on evolutionary diversification. Am. Nat. 2000, 155, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, J.N.; Kassen, R. On the experimental evolution of specialization and diversity in heterogeneous environments. Ecol. Lett. 2007, 10, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Rundle, H.D.; Nosil, P. Ecological speciation. Ecol. Lett. 2005, 8, 336–352. [Google Scholar] [CrossRef]
- Van Der Wurff, A.W.G.; Boivin, M.-E.Y.; van Den Brink, P.J.; Kools, S.A.E.; van Megen, H.; Riksen, J.; Kammenga, J. Type of disturbance and ecological history determine structural stability. Ecol. Appl. 2007, 17, 190–202. [Google Scholar] [CrossRef]
- Azarbad, H.; Laskowski, R.; van Gestel, C.A.M.; van Straalen, N.M.; Nikiel, K.; Röling, W.F.M.; Niklińska, M. Susceptibility to additional stressors in metal-tolerant soil microbial communities from two pollution gradients. Appl. Soil Ecol. 2016, 98, 233–242. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Bardgett, R.D.; Cook, R.; Christensen, S.; Ekelund, F.; Sorensen, S.J.; Bååth, E.; Bloem, J.; de Ruiter, P.C.; et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: An examination of the biodiversity–ecosystem function relationship. Oikos 2000, 90, 279–294. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Bonkowski, M.; Roy, J.; Ritz, K. Functional stability, substrate utilisation and biological indicators of soils following environmental impacts. Appl. Soil Ecol. 2001, 16, 49–61. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Kuan, H.L.; Ritz, K.L.; Glover, A.; McCaig, A.E.; Fenwick, C. The relationship between microbial community structure and functional stability, tested experimentally in an upland pasture soil. Microb. Ecol. 2004, 47, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Kuan, H.L.; Hallett, P.D.; Griffiths, B.S.; Gregory, A.S.; Watts, C.W.; Whitmore, A.P. The biological and physical stability and resilience of a selection of Scottish soils to stresses. Eur. J. Soil Sci. 2007, 58, 811–821. [Google Scholar] [CrossRef]
- Gregory, A.S.; Watts, C.W.; Griffiths, B.S.; Hallett, P.D.; Kuan, H.L.; Whitmore, A.P. The effect of long-term soil management on the physical and biological resilience of a range of arable and grassland soils in England. Geoderma 2009, 153, 172–185. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, H.; Wang, H.; Yin, R.; Hallett, P.D.; Griffiths, B.S.; Daniell, T.J. Does microbial habitat or community structure drive the functional stability of microbes to stresses following re-vegetation of a severely degraded soil? Soil Biol. Biochem. 2010, 49, 850–859. [Google Scholar] [CrossRef]
- Tobor-Kapłon, M.A.; Bloem, J.; de Ruiter, P.C. Functional stability of microbial communities from long-term stressed soils to additional disturbance. Environ. Toxicol. Chem. 2006, 25, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Newman, J.A.; Silverman, B.W.; Turner, S.L.; Lilley, A.K. The contribution of species richness and composition to bacterial services. Nature 2005, 436, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Langenheder, S.; Bulling, M.T.; Solan, M.; Prosser, J.I. Bacterial biodiversity-ecosystem functioning relations are modified by environmental complexity. PLoS ONE 2010, 5, e10834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindström, E.S.; Feng, X.M.; Granél, W.; Kritzberg, E.S. The interplay between bacterial community composition and the environment determining function of inland water bacteria. Limnol. Oceanogr. 2010, 55, 2052–2060. [Google Scholar]
- Naeem, S.; Li, S.B. Biodiversity enhances ecosystem reliability. Nature 1997, 390, 507–509. [Google Scholar] [CrossRef]
- Wertz, S.; Degrange, V.; Prosser, J.I.; Poly, F.; Commeaux, C.; Guillaumaud, N.; Le Roux, X. Decline of soil microbial diversity does not influence the resistance and resilience of key soil microbial functional groups following a model disturbance. Environ. Microbiol. 2007, 9, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Walker, B.H.; Hobbs, R.J.; Hooper, D.U.; Lawton, J.H.; Sala, O.E.; Tilman, D. Biotic control over the functioning of ecosystems. Science 1997, 277, 500–504. [Google Scholar] [CrossRef]
- Luck, G.W.; Daily, G.C.; Ehrlich, P.R. Population diversity and ecosystem services. Trends Ecol. Evol. 2003, 18, 331–336. [Google Scholar] [CrossRef]
- Girvan, M.S.; Campbell, C.D.; Killham, K.; Prosser, J.I.; Glover, L.A. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 2005, 7, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Keiser, A.D.; Strickland, M.S.; Fierer, N.; Bradford, M.A. The effect of resource history on the functioning of soil microbial communities is maintained across time. Biogeosciences 2011, 8, 1477–1486. [Google Scholar] [CrossRef]
- Garbeva, P.; Postma, J.; van Veen, J.A.; van Elsas, J.D. Effect of aboveground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ. Microbiol. 2006, 8, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Heemsbergen, D.A.; Berg, M.P.; Loreau, M.; van Hal, J.R.; Faber, J.H.; Verhoef, H.A. Biodiversity effects on soil processes explained by interspecific functional dissimilarity. Science 2004, 306, 1019. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, K.; Müller, A.K.; Christensen, S.; Bloem, J.; Sørensen, S.J. The effect of Tylosin as a Disturbance on the Soil Microbial Communities. Soil Biol. Biochem. 2001, 33, 2061–2071. [Google Scholar] [CrossRef]
- Velasco, A.G.V.; Probanza, A.; Gutierrez Mañero, F.J.G.; Treviño, A.C.; Moreno, J.M.; Garcia, J.A.L. Effect of fire and retardant on soil microbial activity and functional diversity in a Mediterranean pasture. Geoderma 2009, 153, 186–193. [Google Scholar] [CrossRef]
- Chaer, G.; Fernandes, M.; Myrold, D.; Bottomley, P. Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microbial. Ecol. 2009, 58, 414–494. [Google Scholar] [CrossRef] [PubMed]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seybold, C.A.; Herrick, J.E.; Brejda, J.J. Soil resilience: A fundamental component of soil quality. Soil Sci. 1999, 164, 224–234. [Google Scholar] [CrossRef]
- De Ruiter, P.C.; Griffiths, B.S.; Moore, J.C. Biodiversity and stability in soil ecosystems: Patterns, processes and the effects of disturbance. In Biodiversity and Ecosystem Functioning. Synthesis and Perspectives; Loreau, M., Naeem, S., Inchausti, P., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 102–113. [Google Scholar]
- Domsch, K.H. Effects of pesticides and heavy metals on biological processes in soil. Plant Soil 1984, 76, 367–378. [Google Scholar] [CrossRef]
- Kandeler, E.; Kampichler, C.; Horak, O. Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fertil. Soils 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Arias-Estévez, M.; Díaz-Raviña, M.; Bååth, E. Bacterial pollution induced community tolerance (PICT) to Cu and interactions with pH in long term polluted vineyard soils. Soil Biol. Biochem. 2011, 43, 2324–2331. [Google Scholar] [CrossRef]
- Röling, W.F.M.; Milner, M.G.; Jones, D.M.; Fratepietro, F.; Swannell, R.P.J.; Daniel, F.; Head, I.M. Bacterial community dynamics and hydrocarbon degradation during a field-scale evaluation of bioremediation on a mudflat beach contaminated with buried oil. Appl. Environ. Microbiol. 2004, 70, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Åkerblom, S.; Bååth, E.; Bringmark, L. Experimentally influenced effects of heavy metal on microbial activity and community structure of forest mor layers. Biol. Fertil. Soils 2007, 44, 79–91. [Google Scholar]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Duncan, L.C. Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance? Soil Biol. Biochem. 2001, 33, 1143–1153. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, C.A.M.; Mol, S. The influence of soil characteristics on cadmium toxicity for Folsomia candida (Collembola: Isotomidae). Pedobiologia 2003, 47, 387–395. [Google Scholar] [CrossRef]
- Royer-Tardif, S.; Bradley, R.L.; Parsons, W.F.J. Evidence that plant diversity and site productivity confer stability to forest floor microbial biomass. Soil Biol. Biochem. 2010, 49, 813–821. [Google Scholar] [CrossRef]
- Luthy, R.G.; Aiken, G.R.; Brusseau, M.L.; Cunningham, S.D.; Gschwend, P.M.; Pignatello, J.J.; Reinhard, M.; Traina, S.J.; Weber, W.J.; Westall, J.C. Sequestration of hydrophobic organic contaminants by geosorbents. Environ. Sci. Technol. 1997, 31, 3341–3347. [Google Scholar] [CrossRef]
- Sandaa, R.A.; Torsvik, V.; Enger, O.; Daae, F.L.; Castberg, T.; Hahn, D. Analysis of bacterial communities in heavy metal contaminated soil at different levels of resolution. FEMS Microbiol. Ecol. 1999, 30, 229–236. [Google Scholar] [CrossRef]


| Terms | Definitions | References |
|---|---|---|
| Adaptation | Increase of genetically encoded traits that enhance the fitness of their bearers. | [53] |
| Community composition | The richness, relative abundance, and phylogenetic structure of taxa in an assemblage. | [11] |
| Community structure | Taxonomic composition of a microbial community; can also refer to the spatiotemporal distribution of taxa. | [53] |
| Costs of tolerance | Any deprivation of fitness-related traits that is a consequence of altered resource allocation involved with adaptation to stress. | [29] |
| Ecological history | Ecological and evolutionary events that have occurred at some point in the past, such as dispersal limitation, drift, priority effects, or selection by past environmental conditions. | [54] |
| Functional redundancy | The ability of one microbial taxon to carry out a process at the same rate as another taxon under the same environmental conditions. | [11] |
| Functional stability | The ability of a microbial community to minimize dynamic fluctuations of a function (such as respiration rate, enzyme activity or functional potential gene structure) and to defy changes in the community after a disturbance. | [13] |
| Resilience | The capacity of a community under stress to persist and maintain or recover their original or new stable state in terms of composition and function. | [55,56,57] |
| Resistance | The degree to which a community withstands changes in the face of disturbance. The ability of a community to maintain population structure and function under a toxicity stress. | [11,55,56,57] |
| Stability | The tendency of a community to return to a stable condition after stress; includes the components of resistance and resilience. | [54] |
| Stress | A deviation from optimal conditions that leads to a reduced growth rate or a cellular damage in result of environmental or internal changes. | [53] |
| Tolerance | The ability of a community to withstand toxic insults inflicted by pollutants on the ecosystem, and survive under the resulting conditions. Tolerance merges aspects of physiological adaptation and resistance of microbial populations in a single concept. | [58] |
| Trade-offs | Negative correlation between two life-history (or other) traits in such a way that an increase of one trait imposes a cost to another. | [59] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarbad, H.; Van Gestel, C.A.M.; Niklińska, M.; Laskowski, R.; Röling, W.F.M.; Van Straalen, N.M. Resilience of Soil Microbial Communities to Metals and Additional Stressors: DNA-Based Approaches for Assessing “Stress-on-Stress” Responses. Int. J. Mol. Sci. 2016, 17, 933. https://doi.org/10.3390/ijms17060933
Azarbad H, Van Gestel CAM, Niklińska M, Laskowski R, Röling WFM, Van Straalen NM. Resilience of Soil Microbial Communities to Metals and Additional Stressors: DNA-Based Approaches for Assessing “Stress-on-Stress” Responses. International Journal of Molecular Sciences. 2016; 17(6):933. https://doi.org/10.3390/ijms17060933
Chicago/Turabian StyleAzarbad, Hamed, Cornelis A. M. Van Gestel, Maria Niklińska, Ryszard Laskowski, Wilfred F. M. Röling, and Nico M. Van Straalen. 2016. "Resilience of Soil Microbial Communities to Metals and Additional Stressors: DNA-Based Approaches for Assessing “Stress-on-Stress” Responses" International Journal of Molecular Sciences 17, no. 6: 933. https://doi.org/10.3390/ijms17060933