Early Postnatal Outcome and Care after in Utero Exposure to Lithium: A Single Center Analysis of a Belgian Tertiary University Hospital
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Lithium Assay
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Biochemical Data
3.3. Neurological Assessment and Lithium TDM
4. Discussion
4.1. Main Findings
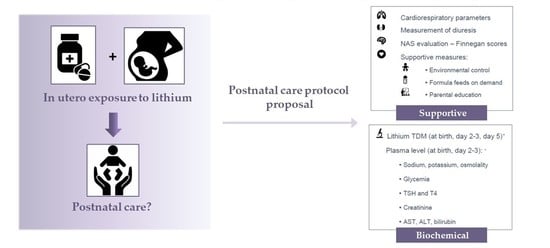
4.2. Proposal of a Postnatal Care Protocol for Neonates after In Utero Exposure to Lithium
- Monitoring cardiorespiratory parameters (heart rate, oxygen saturation, respiration rate)
- Measurement of diuresis to detect polyuria
- Evaluation of (risk for) NAS using the (modified) Finnegan score, every 3 to 4 h.
- Non-pharmacological supportive management (as part of the general NAS approach) [51]:
- Environmental control (swaddling, low-stimulus environment, …)
- Formula feeding on demand
- Parental education
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diav-Citrin, O.; Shechtman, S.; Tahover, E.; Finkel-Pekarsky, V.; Arnon, J.; Kennedy, D.; Erebara, A.; Einarson, A.; Ornoy, A. Pregnancy outcome following in utero exposure to lithium: A prospective, comparative, observational study. Am. J. Psychiatry 2014, 171, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Firth, J.; Vieta, E. Bipolar Disorder. N. Engl. J. Med. 2020, 383, 58–66. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Depression in Adults: Recognition and Management. Clinical Guideline [CG90]. Available online: https://www.nice.org.uk/guidance/cg90 (accessed on 18 June 2022).
- Yonkers, K.A.; Wisner, K.L.; Stowe, Z.; Leibenluft, E.; Cohen, L.; Miller, L.; Manber, R.; Viguera, A.; Suppes, T.; Altshuler, L. Management of bipolar disorder during pregnancy and the postpartum period. Am. J. Psychiatry 2004, 161, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.-P.; Highet, N. The Expert Working Group Mental Health Care in the Perinatal Period: Australian Clinical Practice Guideline. Available online: https://www.cope.org.au/wp-content/uploads/2018/05/COPE-Perinatal-MH-Guideline_Final-2018.pdf (accessed on 7 August 2022).
- National Institute for Health and Care Excellence. Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance. Clinical Guideline [CG192]. Available online: https://www.nice.org.uk/guidance/cg192 (accessed on 18 June 2022).
- McAllister-Williams, R.H.; Baldwin, D.S.; Cantwell, R.; Easter, A.; Gilvarry, E.; Glover, V.; Green, L.; Gregoire, A.; Howard, L.M.; Jones, I.; et al. British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. J. Psychopharmacol. 2017, 31, 519–552. [Google Scholar] [CrossRef] [PubMed]
- Wesseloo, R.; Wierdsma, A.I.; van Kamp, I.L.; Munk-Olsen, T.; Hoogendijk, W.J.G.; Kushner, S.A.; Bergink, V. Lithium dosing strategies during pregnancy and the postpartum period. Br. J. Psychiatry 2017, 211, 31–36. [Google Scholar] [CrossRef]
- Poels, E.M.P.; Bijma, H.H.; Galbally, M.; Bergink, V. Lithium during pregnancy and after delivery: A review. Int. J. Bipolar Disord. 2018, 6, 26. [Google Scholar] [CrossRef]
- Fornaro, M.; Maritan, E.; Ferranti, R.; Zaninotto, L.; Miola, A.; Anastasia, A.; Murru, A.; Sole, E.; Stubbs, B.; Carvalho, A.F.; et al. Lithium Exposure During Pregnancy and the Postpartum Period: A Systematic Review and Meta-Analysis of Safety and Efficacy Outcomes. Am. J. Psychiatry 2020, 177, 76–92. [Google Scholar] [CrossRef]
- Tondo, L.; Alda, M.; Bauer, M.; Bergink, V.; Grof, P.; Hajek, T.; Lewitka, U.; Licht, R.W.; Manchia, M.; Muller-Oerlinghausen, B.; et al. Clinical use of lithium salts: Guide for users and prescribers. Int. J. Bipolar Disord. 2019, 7, 16. [Google Scholar] [CrossRef]
- Alda, M. Lithium in the treatment of bipolar disorder: Pharmacology and pharmacogenetics. Mol. Psychiatry 2015, 20, 661–670. [Google Scholar] [CrossRef]
- Ochoa, E.L.M. Lithium as a Neuroprotective Agent for Bipolar Disorder: An Overview. Cell Mol. Neurobiol. 2022, 42, 85–97. [Google Scholar] [CrossRef]
- Newport, D.J.; Viguera, A.C.; Beach, A.J.; Ritchie, J.C.; Cohen, L.S.; Stowe, Z.N. Lithium placental passage and obstetrical outcome: Implications for clinical management during late pregnancy. Am. J. Psychiatry 2005, 162, 2162–2170. [Google Scholar] [CrossRef]
- Munk-Olsen, T.; Liu, X.; Viktorin, A.; Brown, H.K.; Di Florio, A.; D’Onofrio, B.M.; Gomes, T.; Howard, L.M.; Khalifeh, H.; Krohn, H.; et al. Maternal and infant outcomes associated with lithium use in pregnancy: An international collaborative meta-analysis of six cohort studies. Lancet Psychiatry 2018, 5, 644–652. [Google Scholar] [CrossRef]
- Schou, M.; Goldfield, M.D.; Weinstein, M.R.; Villeneuve, A. Lithium and pregnancy. I. Report from the Register of Lithium Babies. Br. Med. J. 1973, 2, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Patorno, E.; Huybrechts, K.F.; Bateman, B.T.; Cohen, J.M.; Desai, R.J.; Mogun, H.; Cohen, L.S.; Hernandez-Diaz, S. Lithium Use in Pregnancy and the Risk of Cardiac Malformations. N. Engl. J. Med. 2017, 376, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Hastie, R.; Tong, S.; Hiscock, R.; Lindquist, A.; Lindstrom, L.; Wikstrom, A.K.; Sundstrom-Poromaa, I. Maternal lithium use and the risk of adverse pregnancy and neonatal outcomes: A Swedish population-based cohort study. BMC Med. 2021, 19, 291. [Google Scholar] [CrossRef]
- Frassetto, F.; Tourneur Martel, F.; Barjhoux, C.E.; Villier, C.; Bot, B.L.; Vincent, F. Goiter in a newborn exposed to lithium in utero. Ann. Pharmacother. 2002, 36, 1745–1748. [Google Scholar] [CrossRef]
- Pinelli, J.M.; Symington, A.J.; Cunningham, K.A.; Paes, B.A. Case report and review of the perinatal implications of maternal lithium use. Am. J. Obstet. Gynecol. 2002, 187, 245–249. [Google Scholar] [CrossRef]
- Zegers, B.; Andriessen, P. Maternal lithium therapy and neonatal morbidity. Eur. J. Pediatr. 2003, 162, 348–349. [Google Scholar] [CrossRef]
- Stals, T.V.; Vanhole, C.; Debeer, A.; Levtchenko, E.; Smits, A. Nephrogenic diabetes insipidus in a preterm neonate after in utero exposure to lithium. Abstract BVK/SBP Congress (Abstract ID Neonatology K16). Belg. J. Paediatr. 2017, 19, 76. [Google Scholar]
- Molenaar, N.M.; Poels, E.M.P.; Robakis, T.; Wesseloo, R.; Bergink, V. Management of lithium dosing around delivery: An observational study. Bipolar Disord. 2021, 23, 49–54. [Google Scholar] [CrossRef]
- van der Lugt, N.M.; van de Maat, J.S.; van Kamp, I.L.; Knoppert-van der Klein, E.A.; Hovens, J.G.; Walther, F.J. Fetal, neonatal and developmental outcomes of lithium-exposed pregnancies. Early Hum. Dev. 2012, 88, 375–378. [Google Scholar] [CrossRef]
- Kozma, C. Neonatal toxicity and transient neurodevelopmental deficits following prenatal exposure to lithium: Another clinical report and a review of the literature. Am. J. Med. Genet. A 2005, 132A, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, L. Neonatal abstinence syndrome: Assessment and pharmacotherapy. In Current Therapy in Neonatal-Perinatal Medicine, 2nd ed.; Nelson, N., Ed.; BC Decker: Hamilton, ON, USA, 1990. [Google Scholar]
- Finnegan, L.P.; Kaltenbach, K. Neonatal abstinence syndrome. In Primary Pediatric Care, 2nd ed.; Hoekelman, R.A., Friedman, S.B., Nelson, N., Seidel, H.M., Eds.; C V Mosby: St Louis, MO, USA, 1992. [Google Scholar]
- Finnegan, L.P.; Connaughton, J.F., Jr.; Kron, R.E.; Emich, J.P. Neonatal abstinence syndrome: Assessment and management. Addict. Dis. 1975, 2, 141–158. [Google Scholar] [PubMed]
- Kocherlakota, P. Neonatal abstinence syndrome. Pediatrics 2014, 134, e547–e561. [Google Scholar] [CrossRef] [PubMed]
- Asti, L.; Magers, J.S.; Keels, E.; Wispe, J.; McClead, R.E., Jr. A quality improvement project to reduce length of stay for neonatal abstinence syndrome. Pediatrics 2015, 135, e1494–e1500. [Google Scholar] [CrossRef]
- Clark, C.T. Psychotropic drug use in perinatal women with bipolar disorder. Semin. Perinatol. 2020, 44, 151230. [Google Scholar] [CrossRef]
- Kupka, R.; Goossens, P.; van Bendegem, M.; Daemen, P.; Daggenvoorde, T.; Daniels, M.; Dols, A.; Hillegers, M.; Hoogelander, A.; ter Kulve, E.; et al. Multidisciplinaire Richtlijn Bipolaire Stoornissen. Derde, Herziene Versie; De Tijdstroom: Utrecht, The Netherlands, 2015. [Google Scholar]
- Deiana, V.; Chillotti, C.; Manchia, M.; Carta, P.; Bocchetta, A.; Ardau, R.; Del Zompo, M. Continuation versus discontinuation of lithium during pregnancy: A retrospective case series. J. Clin. Psychopharmacol. 2014, 34, 407–410. [Google Scholar] [CrossRef]
- Rosso, G.; Albert, U.; Di Salvo, G.; Scata, M.; Todros, T.; Maina, G. Lithium prophylaxis during pregnancy and the postpartum period in women with lithium-responsive bipolar I disorder. Arch. Womens Ment. Health 2016, 19, 429–432. [Google Scholar] [CrossRef]
- Schoot, T.S.; Molmans, T.H.J.; Grootens, K.P.; Kerckhoffs, A.P.M. Systematic review and practical guideline for the prevention and management of the renal side effects of lithium therapy. Eur. Neuropsychopharmacol. 2020, 31, 16–32. [Google Scholar] [CrossRef]
- Christensen, B.M.; Zuber, A.M.; Loffing, J.; Stehle, J.C.; Deen, P.M.; Rossier, B.C.; Hummler, E. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2011, 22, 253–261. [Google Scholar] [CrossRef]
- Robben, J.H.; Knoers, N.V.; Deen, P.M. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am. J. Physiol. Renal Physiol. 2006, 291, F257–F270. [Google Scholar] [CrossRef] [PubMed]
- Rej, S.; Pira, S.; Marshe, V.; Do, A.; Elie, D.; Looper, K.J.; Herrmann, N.; Muller, D.J. Molecular mechanisms in lithium-associated renal disease: A systematic review. Int. Urol. Nephrol. 2016, 48, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, E.M.; Hobbs, J.F.; Goldsmith, D.I. Nephrogenic diabetes insipidus in transplacental lithium intoxication. J. Pediatr. 1979, 94, 493–495. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Hale, T.W.; Rowe, H.E. Medications and Mothers Milk; Springers Publishing Company, LLC: New York, NY, USA, 2017. [Google Scholar]
- Galbally, M.; Snellen, M.; Walker, S.; Permezel, M. Management of antipsychotic and mood stabilizer medication in pregnancy: Recommendations for antenatal care. Aust. N. Z. J. Psychiatry 2010, 44, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Galbally, M.; Bergink, V.; Vigod, S.N.; Buist, A.; Boyce, P.; Chandra, P.; Kohan, R.; Howard, L.M. Breastfeeding and lithium: Is breast always best? Lancet Psychiatry 2018, 5, 534–536. [Google Scholar] [CrossRef]
- Imaz, M.L.; Torra, M.; Soy, D.; Garcia-Esteve, L.; Martin-Santos, R. Clinical Lactation Studies of Lithium: A Systematic Review. Front. Pharmacol. 2019, 10, 1005. [Google Scholar] [CrossRef]
- Imaz, M.L.; Langohr, K.; Torra, M.; Soy, D.; Garcia-Esteve, L.; Martin-Santos, R. Neonatal Feeding Trajectories in Mothers with Bipolar Disorder Taking Lithium: Pharmacokinetic Data. Front. Pharmacol. 2021, 12, 752022. [Google Scholar] [CrossRef]
- Heinonen, E.; Totterman, K.; Back, K.; Sarman, I.; Svedenkrans, J.; Forsberg, L. Lithium use during breastfeeding was safe in healthy full-term infants under strict monitoring. Acta Paediatr. 2022. [Google Scholar] [CrossRef]
- Imaz, M.L.; Soy, D.; Torra, M.; Garcia-Esteve, L.; Soler, C.; Martin-Santos, R. Case Report: Clinical and Pharmacokinetic Profile of Lithium Monotherapy in Exclusive Breastfeeding. A Follow-Up Case Series. Front. Pharmacol. 2021, 12, 647414. [Google Scholar] [CrossRef]
- McKnight, S.; Coo, H.; Davies, G.; Holmes, B.; Newman, A.; Newton, L.; Dow, K. Rooming-in for Infants at Risk of Neonatal Abstinence Syndrome. Am. J. Perinatol. 2016, 33, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.I.; Mauer-Vakil, D.; Coo, H.; Newton, L.; Wilkerson, E.; McKnight, S.; Brogly, S.B. Rooming-in for Infants at Risk for Neonatal Abstinence Syndrome: Outcomes 5 Years following Its Introduction as the Standard of Care at One Hospital. Am. J. Perinatol. 2020, 39, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Smirk, C.L.; Bowman, E.; Doyle, L.W.; Kamlin, C.O. How long should infants at risk of drug withdrawal be monitored after birth? J. Paediatr. Child Health 2014, 50, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Mangat, A.K.; Schmolzer, G.M.; Kraft, W.K. Pharmacological and non-pharmacological treatments for the Neonatal Abstinence Syndrome (NAS). Semin. Fetal Neonatal Med. 2019, 24, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Raffaeli, G.; Cavallaro, G.; Allegaert, K.; Wildschut, E.D.; Fumagalli, M.; Agosti, M.; Tibboel, D.; Mosca, F. Neonatal Abstinence Syndrome: Update on Diagnostic and Therapeutic Strategies. Pharmacotherapy 2017, 37, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, L.; Adler, M.; Romer Ek, I.; Ljungdahl, M.; Naver, L.; Gustafsson, L.L.; Berglund, G.; Chotigasatien, A.; Hammar, U.; Bohm, B.; et al. Maternal mood disorders and lithium exposure in utero were not associated with poor cognitive development during childhood. Acta Paediatr. 2018, 107, 1379–1388. [Google Scholar] [CrossRef]
- Schou, M. What happened later to the lithium babies? A follow-up study of children born without malformations. Acta Psychiatr. Scand. 1976, 54, 193–197. [Google Scholar] [CrossRef]
- Jacobson, S.J.; Jones, K.; Johnson, K.; Ceolin, L.; Kaur, P.; Sahn, D.; Donnenfeld, A.E.; Rieder, M.; Santelli, R.; Smythe, J.; et al. Prospective multicentre study of pregnancy outcome after lithium exposure during first trimester. Lancet 1992, 339, 530–533. [Google Scholar] [CrossRef]
- Poels, E.M.P.; Schrijver, L.; White, T.J.H.; Roza, S.J.; Zarchev, M.G.; Bijma, H.; Honig, A.; van Kamp, I.L.; Hoogendijk, W.J.G.; Kamperman, A.M.; et al. The effect of prenatal lithium exposure on the neuropsychological development of the child. Bipolar Disord. 2022, 24, 310–319. [Google Scholar] [CrossRef]





| Study ID Mother | Primary Diagnosis | Pregnancy Complications | Perinatal Lithium Dose (mg/day) | Perinatal Lithium Dose (mg/kg) | Lithium TDM at Delivery (mmol/L) | Concomitant Medication | |
|---|---|---|---|---|---|---|---|
| 1 | Bipolar disorder | Hypertension | 600 | 7.20 | 0.50 | levothyroxine, quetiapine | |
| 2 | Bipolar disorder | Gestational diabetes mellitus | 1200 | 15.52 | 0.60 | levothyroxine, quetiapine, acetylsalicylic acid, insulin, olanzapine | |
| 3 | Bipolar disorder | Gestational diabetes mellitus | 1500 | 20.32 | 0.84 | levothyroxine, haloperidol, lamotrigine | |
| 4 | Bipolar disorder | Gestational diabetes mellitus | 1000 | 10.48 | 0.72 | levothyroxine, lorazepam, olanzapine | |
| 5 | Bipolar disorder | - | 1400 | 11.57 | 0.64 | aripiprazole, acetylsalicylic acid | |
| 6 | Bipolar disorder | - | 500 | 6.57 | 0.63 | levothyroxine | |
| 7 | Bipolar disorder | PPROM, shortened cervix | 168 | 3.57 | 0.18 | nifedipine | |
| 8 | Bipolar disorder | - | 625 | 9.08 | 0.54 | levothyroxine | |
| 9 | Bipolar disorder | - | 1000 | 10.89 | 0.58 | - | |
| 10 | Bipolar disorder | Intrahepatic cholestasis of pregnancy Gestational diabetes mellitus | 1000 | 10.47 | 0.92 | levothyroxine, quetiapine, insulin | |
| Median | 1000 | 10.48 | 0.62 | ||||
| IQR | 600–1200 | 7.20–11.57 | 0.54–0.72 |
| Study ID Neonate | Sex | GA (Weeks) | BW (Grams) | Apgar 1 | Apgar 5 | Apgar 10 | pH * | Congenital Malformation |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 38 | 2990 | 5 | 6 | 9 | 7.25 | - |
| 2 | F | 37 | 3440 | 9 | 9 | 10 | - | - |
| 3 | M | 39 | 3010 | 9 | 9 | 10 | - | - |
| 4 | F | 37 | 2200 | 9 | 10 | 10 | 7.27 | - |
| 5 | M | 37 | 2620 | 9 | 10 | 10 | 7.23 | - |
| 6 | F | 36 | 2950 | 2 | 4 | 8 | 7.13 | - |
| 7 | F | 29 | 1200 | 6 | 4 | 5 | 7.31 | CDH ** |
| 8 | F | 40 | 3100 | 9 | 9 | 9 | 7.21 | - |
| 9 | M | 41 | 4805 | 8 | 9 | 9 | - | - |
| 10 | M | 36 | 3505 | 10 | 10 | 10 | 7.15 | - |
| Median | 37 | 3000 | 9 | 9 | 9.5 | 7.23 | ||
| IQR | 36–39 | 2620–3440 | 6–9 | 6–10 | 9–10 | 7.17–7.27 |
| Study ID Neonate | Need for Respiratory Support | Duration of Respiratory Support (Days) | Diet | Time to Full Enteral Feeding (Days) | Time to Full Oral Feeding (Days) | Length of Stay (Days) | Lithium TDM at Birth (mmol/L) |
|---|---|---|---|---|---|---|---|
| 1 | Yes, CPAP | 1 | Formula | 0 | 7 | 9 | 0.50 |
| 2 | No | 0 | Formula | 5 | 5 | 10 | 0.62 |
| 3 | No | 0 | Formula | 0 | 0 | 8 | 0.92 |
| 4 | No | 0 | Formula | 0 | 0 | 20 | 0.52 |
| 5 | No | 0 | Formula | 0 | 0 | 8 | 0.60 |
| 6 | Yes, CPAP | 1 | Formula | 3 | 3 | 8 | 0.67 |
| 7 | Yes, IMV | 37 | Formula | - | - | 37 | - |
| 8 | No | 0 | Formula | 0 | 0 | 6 | 2.09 |
| 9 | No | 0 | Formula | 0 | 0 | 6 | - |
| 10 | No | 0 | Formula | 0 | 0 | 12 | 0.74 |
| Median | 0 | 0 | 0 | 8.5 | 0.65 | ||
| IQR | 0–1 | 0–0.75 | 0–3.50 | 8–12 | 0.56–0.83 |
| Study ID Neonate | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochemical Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Median | IQR |
| Sodium (mEq/L) | 135.50 | 141.80 | - | 139.00 | 138.20 | 138.30 | 139.20 | 137.90 | 139.50 | 136.70 | 138.30 | 137.60–139.28 |
| Potassium (mEq/L) | 6.03 | 5.16 | - | 6.18 | 5.73 | 4.49 | 4.52 | 5.67 | 5.28 | 4.91 | 5.28 | 4.81–5.81 |
| AST * (U/L) | 44.00 | - | - | 29.00 | 31.00 | - | - | - | - | - | 31.00 | 29.50–40.75 |
| ALT ** (U/L) | 6.00 | - | - | 5.00 | 6.00 | - | <5.00 | - | - | - | 5.50 | 3.75–6.00 |
| Creatinemia (mg/dL) | 0.74 | 0.91 | - | 0.61 | 0.73 | 0.81 | 0.67 | 0.73 | 0.64 | 0.65 | 0.73 | 0.65–0.76 |
| FT4 *** (pmol/L) | 14.10 | - | 15.70 | 14.00 | 16.60 | 16.80 | 14.30 | 15.00 | 13.12 # | 15.60 | 15.00 | 14.08–15.93 |
| TSH **** (mIU/L) | 8.48 | 8.32 | 14.56 | 6.91 | 31.82 | 17.61 | 10.25 | 7.97 | 13.67 | 14.80 | 11.96 | 8.32–14.80 |
| Glycemia, mean (mg/dL) | 61.20 | 56.33 | 64.25 | 62.50 | 57.25 | 59.75 | 64.40 | 113.00 | 66.00 | 67.80 | 63.38 | 59.75–66.00 |
| Glycemia, maximum (mg/dL) | 73.00 | 62.00 | 82.00 | 70.00 | 73.00 | 60.00 | 112.00 | 113.00 | 66.00 | 80.00 | 73.00 | 66.00–82.00 |
| Glycemia, minimum (mg/dL) | 46.00 | 56.00 | 56.00 | 50.00 | 41.00 | 44.00 | 20.00 | 113.00 | 66.00 | 43.00 | 48.00 | 43.00–56.00 |
| Study ID Neonate | Highest Finnegan Score | Timing of Highest Finnegan Score | Neonatal Lithium TDM at Birth (mmol/L) | Neonate/Mother TDM Ratio | Clinical Neurological Examination, or Cerebral Ultrasound |
|---|---|---|---|---|---|
| 1 | 6 | Day 1 | 0.50 | 1.00 | Floppy, irritable, mild tremor |
| 2 | 4 | Day 2–3 | 0.62 | 1.03 | Sleepy, feeding difficulties |
| 3 | - | - | 0.92 | 1.10 | Normal |
| 4 | 7 | Day 3 | 0.52 | 0.72 | Floppy, irritable, sleepy, feeding difficulties |
| 5 | - | - | 0.60 | 0.94 | Normal |
| 6 | 4 | Day 1 | 0.67 | 1.06 | Floppy, horizontal nystagmus until day 2, slow grasping reflex, feeding difficulties |
| 7 | - | - | - | - | Grade 3 intraventricular haemorrhage |
| 8 | 1 | Day 3 | 2.09 | 3.87 | Normal |
| 9 | - | - | - | - | Normal |
| 10 | 3 | Day 1–2 | 0.74 | 0.80 | Normal |
| Median | 4 | 0.65 | 1.02 | ||
| IQR | 3–6 | 0.56–0.83 | 0.87–1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torfs, M.; Hompes, T.; Ceulemans, M.; Van Calsteren, K.; Vanhole, C.; Smits, A. Early Postnatal Outcome and Care after in Utero Exposure to Lithium: A Single Center Analysis of a Belgian Tertiary University Hospital. Int. J. Environ. Res. Public Health 2022, 19, 10111. https://doi.org/10.3390/ijerph191610111
Torfs M, Hompes T, Ceulemans M, Van Calsteren K, Vanhole C, Smits A. Early Postnatal Outcome and Care after in Utero Exposure to Lithium: A Single Center Analysis of a Belgian Tertiary University Hospital. International Journal of Environmental Research and Public Health. 2022; 19(16):10111. https://doi.org/10.3390/ijerph191610111
Chicago/Turabian StyleTorfs, Marlien, Titia Hompes, Michael Ceulemans, Kristel Van Calsteren, Christine Vanhole, and Anne Smits. 2022. "Early Postnatal Outcome and Care after in Utero Exposure to Lithium: A Single Center Analysis of a Belgian Tertiary University Hospital" International Journal of Environmental Research and Public Health 19, no. 16: 10111. https://doi.org/10.3390/ijerph191610111