Vat Photopolymerization 3D Printing of Hydrogels with Re-Adjustable Swelling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of the Photopolymerizable Resin and Optimization of the 3D Printing Parameters
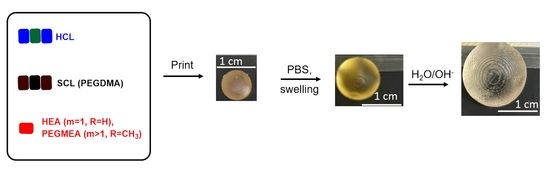
2.2. 3D Printed Part Swelling: Toward a Selective Rupture of the Hydrolysable Crosslinking Agents
2.3. Proof of Concept of the Methodology: Preparation of 3D Printed Hydrogels with Tunable Swelling and Complex Structures
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Photopolymerizable Resin Formulations for 3D Printing
4.3. Selection of the 3D Printing and Post-Processing Parameters
4.4. Swelling Tests Performed on the 3D Printed Parts
4.5. Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagac, M.; Hajnys, J.; Ma, Q.-P.; Jancar, L.; Jansa, J.; Stefek, P.; Mesicek, J. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3D printing. Polymers 2021, 13, 598. [Google Scholar] [CrossRef]
- Nechausov, S.; Ivanchenko, A.; Morozov, O.; Miriyev, A.; Must, I.; Platnieks, O.; Jurinovs, M.; Gaidukovs, S.; Aabloo, A.; Kovač, M. Effects of ionic liquids and dual curing on vat photopolymerization process and properties of 3d-printed ionogels. Addit. Manuf. 2022, 56, 102895. [Google Scholar] [CrossRef]
- Borrello, J.; Nasser, P.; Iatridis, J.C.; Costa, K.D. 3D printing a mechanically-tunable acrylate resin on a commercial DLP-SLA printer. Addit. Manuf. 2018, 23, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Park, Y.; Huh, K.M.; Kang, S.-W. Applications of biomaterials in 3D cell culture and contributions of 3D cell culture to drug development and basic biomedical research. Int. J. Mol. Sci. 2021, 22, 2491. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.; Shalumon, K.; Chen, J.-P. Natural polymers based hydrogels for cell culture applications. Curr. Med. Chem. 2020, 27, 2734–2776. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Shimamoto, G.; Maeda, S. Conical frustum gel driven by the Marangoni effect for a motor without a stator. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125561. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef]
- Sánchez-Correa, F.; Vidaurre-Agut, C.; Serrano-Aroca, Á.; Campillo-Fernández, A.J. Poly (2-hydroxyethyl acrylate) hydrogels reinforced with graphene oxide: Remarkable improvement of water diffusion and mechanical properties. J. Appl. Polym. Sci. 2018, 135, 46158. [Google Scholar] [CrossRef]
- Wu, H.; Chen, P.; Yan, C.; Cai, C.; Shi, Y. Four-dimensional printing of a novel acrylate-based shape memory polymer using digital light processing. Mater. Des. 2019, 171, 107704. [Google Scholar] [CrossRef]
- Ni, R.; Qian, B.; Liu, C.; Liu, X.; Qiu, J. A cross-linking strategy with moderated pre-polymerization of resin for stereolithography. RSC Adv. 2018, 8, 29583–29588. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Wang, W.; Vermonden, T.; Heath, F.; Hennink, W.E.; Alexander, C.; Shakesheff, K.M.; Howdle, S.M. Thermoresponsive and photocrosslinkable PEGMEMA-PPGMA-EGDMA copolymers from a one-step ATRP synthesis. Biomacromolecules 2009, 10, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Sujan, M.I.; Sarkar, S.D.; Sultana, S.; Bushra, L.; Tareq, R.; Roy, C.K.; Azam, M.S. Bi-functional silica nanoparticles for simultaneous enhancement of mechanical strength and swelling capacity of hydrogels. RSC Adv. 2020, 10, 6213–6222. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalossaka, L.M.; Sena, G.; Barter, L.M.; Myant, C. 3D printing hydrogels for the fabrication of soilless cultivation substrates. Appl. Mater. Today 2021, 24, 101088. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics. J. Mater. Sci. Mater. Med. 2020, 31, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Ehrenhofer, A.; Elstner, M.; Wallmersperger, T. Normalization of hydrogel swelling behavior for sensoric and actuatoric applications. Sens. Actuators B Chem. 2018, 255, 1343–1353. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Zhang, J.; Zheng, Y. Constitutive modeling for polymer hydrogels: A new perspective and applications to anisotropic hydrogels in free swelling. Eur. J. Mech.-A Solids 2015, 54, 171–186. [Google Scholar] [CrossRef]
- Liz-Basteiro, P.; Sanz-Horta, R.; Reviriego, F.; Martínez-Campos, E.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A. High Resolution Molds, Sacrificial in Aqueous Media, Obtained by Vat Photopolymerization 3D Printing. Addit. Manuf. Submitt. 2023; under review. [Google Scholar]
- Wu, X.; Xu, C.; Zhang, Z. Flexible film separation analysis of LCD based mask stereolithography. J. Mater. Process. Technol. 2021, 288, 116916. [Google Scholar] [CrossRef]
- Wu, X.; Xu, C.; Zhang, Z. Preparation and optimization of Si3N4 ceramic slurry for low-cost LCD mask stereolithography. Ceram. Int. 2021, 47, 9400–9408. [Google Scholar] [CrossRef]
- Zhu, N.; Hou, Y.; Yang, W.; Wen, G.; Zhong, C.; Wang, D.; Liu, Y.; Zhang, L. Preparation of complex SiOC ceramics by a novel photocurable precursor with liquid crystal display (LCD) 3D printing technology. J. Eur. Ceram. Soc. 2022, 42, 3204–3212. [Google Scholar] [CrossRef]
- Li, J.; Cui, Y.; Qin, K.; Yu, J.; Guo, C.; Yang, J.; Zhang, C.; Jiang, D.; Wang, X. Synthesis and properties of a low-viscosity UV-curable oligomer for three-dimensional printing. Polym. Bull. 2016, 73, 571–585. [Google Scholar] [CrossRef]
- Tu, J.; Makarian, K.; Alvarez, N.J.; Palmese, G.R. Formulation of a model resin system for benchmarking processing-property relationships in high-performance photo 3D printing applications. Materials 2020, 13, 4109. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mao, Q.; Yin, J.; Wang, Y.; Fu, J.; Huang, Y. Theoretical prediction and experimental validation of the digital light processing (DLP) working curve for photocurable materials. Addit. Manuf. 2021, 37, 101716. [Google Scholar] [CrossRef]
- Arias-Ferreiro, G.; Ares-Pernas, A.; Dopico-García, M.S.; Lasagabaster-Latorre, A.; Abad, M.-J. Photocured conductive PANI/acrylate composites for digital light processing. Influence of HDODA crosslinker in rheological and physicochemical properties. Eur. Polym. J. 2020, 136, 109887. [Google Scholar] [CrossRef]
- Arias-Ferreiro, G.; Ares-Pernas, A.; Lasagabáster-Latorre, A.; Aranburu, N.; Guerrica-Echevarria, G.; Dopico-García, M.S.; Abad, M.-J. Printability study of a conductive polyaniline/acrylic formulation for 3d printing. Polymers 2021, 13, 2068. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Prud, R.K.; Aksay, I.A. Cure depth in photopolymerization: Experiments and theory. J. Mater. Res. 2001, 16, 3536–3544. [Google Scholar] [CrossRef] [Green Version]
- Kowsari, K.; Zhang, B.; Panjwani, S.; Chen, Z.; Hingorani, H.; Akbari, S.; Fang, N.X.; Ge, Q. Photopolymer formulation to minimize feature size, surface roughness, and stair-stepping in digital light processing-based three-dimensional printing. Addit. Manuf. 2018, 24, 627–638. [Google Scholar] [CrossRef]
- Garcia, C.; Gallardo, A.; Lo, D.; Elvira, C.; Azzahti, A.; Lopez-Martinez, E.; Cortajarena, A.L.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Smart pH-responsive antimicrobial hydrogel scaffolds prepared by additive manufacturing. ACS Appl. Bio. Mater. 2018, 1, 1337–1347. [Google Scholar] [CrossRef]
- Zhang, Y.; An, D.; Pardo, Y.; Chiu, A.; Song, W.; Liu, Q.; Zhou, F.; McDonough, S.P.; Ma, M. High-water-content and resilient PEG-containing hydrogels with low fibrotic response. Acta biomaterialia 2017, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Preobrazhenskiy, I.I.; Putlyaev, V.I. The ability to control swelling and degradation processes of hydrogels based on a mixture of PEGMA/PEGDA monomers. Mendeleev Commun. 2023, 33, 83–85. [Google Scholar] [CrossRef]
- Pradas, M.M.; Ribelles, J.G.; Aroca, A.S.; Ferrer, G.G.; Antón, J.S.; Pissis, P. Porous poly (2-hydroxyethyl acrylate) hydrogels. Polymer 2001, 42, 4667–4674. [Google Scholar] [CrossRef]
- Nuvoli, D.; Alzari, V.; Nuvoli, L.; Rassu, M.; Sanna, D.; Mariani, A. Synthesis and characterization of poly (2-hydroxyethylacrylate)/β-cyclodextrin hydrogels obtained by frontal polymerization. Carbohydr. Polym. 2016, 150, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Talu, M.; Özgün, H. Alkaline hydrolysis of poly (ethyl acrylate) and styrene-ethyl acrylate copolymer. Eur. Polym. J. 1990, 26, 5–7. [Google Scholar] [CrossRef]
- Garcıa, D.; Escobar, J.; Bada, N.; Casquero, J.; Hernáez, E.; Katime, I. Synthesis and characterization of poly (methacrylic acid) hydrogels for metoclopramide delivery. Eur. Polym. J. 2004, 40, 1637–1643. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mu, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Arias-Ferreiro, G.; Lasagabáster-Latorre, A.; Ares-Pernas, A.; Ligero, P.; García-Garabal, S.M.; Dopico-García, M.S.; Abad, M.-J. Lignin as a High-Value Bioaditive in 3D-DLP Printable Acrylic Resins and Polyaniline Conductive Composite. Polymers 2022, 14, 4164. [Google Scholar] [CrossRef]
- Gojzewski, H.; Guo, Z.; Grzelachowska, W.; Ridwan, M.; Hempenius, M.; Grijpma, D.; Vancso, G. Layer-by-layer printing of photopolymers in 3D: How weak is the interface? ACS Appl. Mater. Interfaces 2020, 12, 8908–8914. [Google Scholar] [CrossRef] [Green Version]









| Sample Label | PEGDMA/HCL/HEA/PEGMEA wt. % | PEGDMA/HCL/HEA/PEGMEA mol % | Crosslinker mol% (PEGDMA + HCL) | Viscosity (Pa·s) for a Shear Rate of 1 s−1 | Dp (µm) | Ec (mJ·cm−2) |
|---|---|---|---|---|---|---|
| PEGDMA7.5-HEA85 | 7.5/7.5/85/0 | 1.8/1.0/97.2/0 | 2.8 | 0.012 ± 0.001 | 311 | 4.89 |
| PEGDMA7.5-HEA60 | 7.5/7.5/60/25 | 2.3/1.2/87.7/8.8 | 3.5 | 0.019 ± 0.001 | 221 | 4.69 |
| PEGDMA7.5-HEA40 | 7.5/7.5/40/45 | 3.0/1.6/75.0/20.4 | 4.6 | 0.026 ± 0.001 | 165 | 4.35 |
| PEGDMA7.5-HEA20 | 7.5/7.5/20/65 | 4.2/2.2/52.4/41.2 | 6.4 | 0.034 ± 0.001 | 105 | 3.49 |
| PEGDMA7.5-HEA0 | 7.5/7.5/0/85 | 6.9/3.6/0/89.5 | 10.5 | 0.041 ± 0.001 | 102 | 3.82 |
| Sample Label | PEGDMA/HCL/HEA/PEGMEA wt. % | PEGDMA/HCL mol % | Viscosity (Pa·s) for a Shear Rate of 1 s−1 | Dp (µm) | Ec (mJ·cm−2) |
|---|---|---|---|---|---|
| PEGDMA2-HEA60 | 2/13/60/25 | 0.6/2.1 | 0.024 ± 0.001 | 221 | 4.69 |
| PEGDMA5-HEA60 | 5/10/60/25 | 1.5/1.6 | |||
| PEGDMA7.5-HEA60 | 7.5/7.5/60/25 | 2.3/1.2 | 0.019 ± 0.001 | 176 | 4.52 |
| PEGDMA10-HEA60 | 10/5/60/25 | 3.1/0.8 | |||
| PEGDMA13-HEA60 | 13/2/60/25 | 4.0/0.3 | |||
| PEGDMA15-HEA60 | 15/0/60/25 | 4.6/0 | 0.012 ± 0.001 | 133 | 5.08 |
| Spheres | Fresh printed parts, diameter (cm) | ||
| SP1: 0.50 | SP2: 0.75 | ||
 SP1 |  SP2 | ||
| PBS (after 48 h), swelling | |||
| SP1 140 ± 1 | SP2 142 ± 2 | ||
 SP1 |  SP2 | ||
| pH 10, 60 °C (after 372 h), swelling | |||
| SP1 853 ± 10 | SP2 820 ± 73 | ||
 SP1 |  SP2 | ||
| Tubes | Fresh printed parts: diameter, wall thickness, length (mm) | ||
| T1: 10, 0.5, 10 | T2: 15, 0.75, 15 | T3: 20, 1, 20 | |
 T1 |  T2 |  T3 | |
| pH 10, 60 °C (after 144 h); swelling | |||
| T1 763 ± 9 | T2 713 ± 15 | T3 748 ± 48 | |
| Seahorse |  | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liz-Basteiro, P.; Reviriego, F.; Martínez-Campos, E.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A. Vat Photopolymerization 3D Printing of Hydrogels with Re-Adjustable Swelling. Gels 2023, 9, 600. https://doi.org/10.3390/gels9080600
Liz-Basteiro P, Reviriego F, Martínez-Campos E, Reinecke H, Elvira C, Rodríguez-Hernández J, Gallardo A. Vat Photopolymerization 3D Printing of Hydrogels with Re-Adjustable Swelling. Gels. 2023; 9(8):600. https://doi.org/10.3390/gels9080600
Chicago/Turabian StyleLiz-Basteiro, Pedro, Felipe Reviriego, Enrique Martínez-Campos, Helmut Reinecke, Carlos Elvira, Juan Rodríguez-Hernández, and Alberto Gallardo. 2023. "Vat Photopolymerization 3D Printing of Hydrogels with Re-Adjustable Swelling" Gels 9, no. 8: 600. https://doi.org/10.3390/gels9080600