Robust Silica–Agarose Composite Aerogels with Interpenetrating Network Structure by In Situ Sol–Gel Process
Abstract
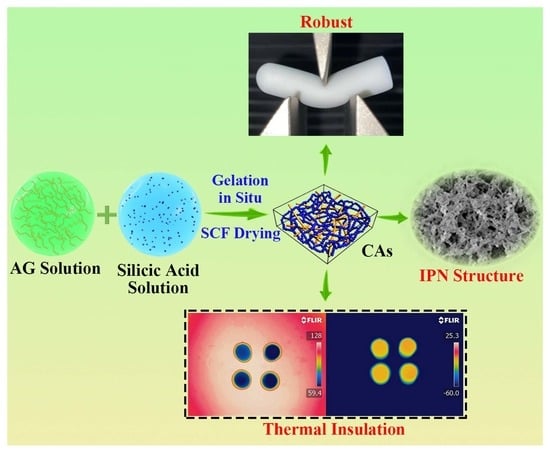
:1. Introduction
2. Results and Discussion
2.1. Microstructures Characterization
2.2. Nitrogen Adsorption–Desorption Test
2.3. Mechanical Properties
2.4. Thermal Insulation Performance
2.5. Thermal Stability
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preprocessing of 732 Cation Exchange Resin
4.3. Preparation of AG–SiO2 Composite Aerogels
4.4. Preparation of AAs
4.5. Preparation of SAs
4.6. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sai, H.; Fu, R.; Xiang, J.; Guan, Y.; Zhang, F. Fabrication of elastic silica-bacterial cellulose composite aerogels with nanoscale interpenetrating network by ultrafast evaporative drying. Compos. Sci. Technol. 2018, 155, 72–80. [Google Scholar] [CrossRef]
- Sai, H.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Zhao, C.; Li, Z.; Li, F.; Zhang, T. Flexible aerogels with interpenetrating network structure of bacterial cellulose–silica composite from sodium silicate precursor via freeze drying process. RSC Adv. 2014, 4, 30453–30461. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ji, C.; Fu, R.; Yang, X.; Wan, Z.; Wen, L.; Song, Q.; Liu, Y.; Wang, Y.; Sai, H. Robust SiO2–Al2O3/Agarose Composite Aerogel Beads with Outstanding Thermal Insulation Based on Coal Gangue. Gels 2022, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Bi, H.; Lin, X.; Cai, L.; Xu, M. Lightweight, Anisotropic, Compressible, and Thermally-Insulating Wood Aerogels with Aligned Cellulose Fibers. Polymers 2020, 12, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, L.; Lyu, Y.; Han, W. Microstructure and Thermal Insulation Property of Silica Composite Aerogel. Materials 2019, 12, 993. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Chen, Z.; Dong, W.; Lin, L.; Zhu, X.; Liu, Q.; Zhang, Y.; Zhai, N.; Zhou, Z.; Wang, Y.; et al. A review of silicon-based aerogel thermal insulation materials: Performance optimization through composition and microstructure. J. Non-Cryst. Solids 2021, 553, 120517–120534. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Naguib, H.E.; Park, C.B. Advances in precursor system for silica-based aerogel production toward improved mechanical properties, customized morphology, and multifunctionality: A review. Adv. Colloid Interface Sci. 2020, 276, 102101–102129. [Google Scholar] [CrossRef]
- Zhang, R.; An, Z.; Zhao, Y.; Zhang, L.; Zhou, P. Nanofibers reinforced silica aerogel composites having flexibility and ultra-low thermal conductivity. Int. J. Appl. Ceram. Technol. 2020, 17, 1531–1539. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Hirashima, H.; Rao, A.V. Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels. Microporous Mesoporous Mater. 2009, 117, 617–626. [Google Scholar] [CrossRef]
- Cai, L.; Shan, G.R. Elastic silica aerogel using methyltrimethoxysilane precusor via ambient pressure drying. J. Porous Mater. 2015, 22, 1455–1463. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, X.H.; Song, H.H.; Guo, K.; Hu, Z.J. Highly flexible silica aerogels derived from methyltriethoxysilane and polydimethylsiloxane. New J. Chem. 2015, 39, 7832–7838. [Google Scholar] [CrossRef]
- Wang, L.; Feng, J.; Jiang, Y.; Li, L.; Feng, J. Elastic methyltrimethoxysilane based silica aerogels reinforced with polyvinylmethyldimethoxysilane. RSC Adv. 2019, 9, 10948–10957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.Q.; Nguyen, B.N.; McCorkle, L.S.; Shonkwiler, B.; Meador, M.A.B. Elastic low density aerogels derived from bis 3-(triethoxysilyl)propyl disulfide, tetramethylorthosilicate and vinyltrimethoxysilane via a two-step process. J. Mater. Chem. 2009, 19, 9054–9062. [Google Scholar] [CrossRef]
- Yun, S.; Luo, H.J.; Gao, Y.F. Low-density, hydrophobic, highly flexible ambientpressure-dried monolithic bridged silsesquioxane aerogels. J. Mater. Chem. A 2015, 3, 3390–3398. [Google Scholar] [CrossRef]
- Zu, G.Q.; Kanamori, K.; Maeno, A.; Kaji, H.; Nakanishi, K. Superflexible Multifunctional Polyvinylpolydimethylsiloxane-Based Aerogels as Efficient Absorbents, Thermal Superinsulators, and Strain Sensors. Angew. Chem. Int. Ed. 2018, 57, 9722–9727. [Google Scholar] [CrossRef] [Green Version]
- Zu, G.Q.; Shimizu, T.; Kanamori, K.; Zhu, Y.; Maeno, A.; Kaji, H.; Shen, J.; Nakanishi, K. Transparent, Superflexible Doubly Cross-Linked Polyvinylpolymethylsiloxane Aerogel Superinsulators via Ambient Pressure Drying. Acs Nano 2018, 12, 521–532. [Google Scholar] [CrossRef]
- Zu, G.Q.; Kanamori, K.; Shimizu, T.; Zhu, Y.; Maeno, A.; Kaji, H.; Nakanishi, K.; Shen, J. Versatile Double-Cross-Linking Approach to Transparent, Machinable, Supercompressible, Highly Bendable Aerogel Thermal Superinsulators. Chem. Mater. 2018, 30, 2759–2770. [Google Scholar] [CrossRef] [Green Version]
- Kamaraj, M.; Kamble, S.; Sonia, S. Facile Synthesis of Silica Composites with Oil Sorption Efficiency from a Vital Agricultural Waste of Corn Stalk Cultivated in Bishoftu, Ethiopia. Adsorpt. Sci. Technol. 2021, 2021, 7205135. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Pasymi, P.; Bindar, Y. An appropriate acid leaching sequence in rice husk ash extraction to enhance the produced green silica quality for sustainable industrial silica gel purpose. J. Taiwan Inst. Chem. E 2021, 122, 51–57. [Google Scholar] [CrossRef]
- Kaya, G.G.; Deveci, H. Modified silica xerogel derived from groundnut hull ash by alkyl-ammonium salt for epoxy nanocomposites: Synergistic effects on thermal stability and flame retardancy. Thermochim. Acta 2020, 689, 178637–178647. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, X.; Zhang, M.; Guo, M. Synthesis of SiO2–Al2O3 composite aerogel from fly ash: A low-cost and facile approach. J. Sol-Gel Sci. Technol. 2020, 93, 281–290. [Google Scholar] [CrossRef]
- Du, A.; Wang, H.Q.; Zhou, B.; Zhang, C.; Wu, X.L.; Ge, Y.T.; Niu, T.T.; Ji, X.J.; Zhang, T.; Zhang, Z.H.; et al. Multifunctional Silica Nanotube Aerogels Inspired by Polar Bear Hair for Light Management and Thermal Insulation. Chem. Mater. 2018, 30, 6849–6857. [Google Scholar] [CrossRef]
- Song, Q.; Miao, C.; Sai, H.; Gu, J.; Wang, M.; Jiang, P.; Wang, Y.; Fu, R.; Wang, Y. Silica-Bacterial Cellulose Composite Aerogel Fibers with Excellent Mechanical Properties from Sodium Silicate Precursor. Gels 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, J.; Petit, D.; Armstrong, J.N.; Hanson, K.; Hamilton, J.; Souza, M.; Zhao, D.; Li, C.; Liu, Y. An all-ceramic, anisotropic, and flexible aerogel insulation material. Nano Lett. 2020, 20, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Markevicius, G.; Ladj, R.; Niemeyer, P.; Budtova, T.; Rigacci, A. Ambient-dried thermal superinsulating monolithic Silica-based aerogels with short cellulosic fibers. J. Mater. Sci. 2016, 52, 2210–2221. [Google Scholar] [CrossRef]
- Slosarczyk, A. Carbon Fiber-Silica Aerogel Composite with Enhanced Structural and Mechanical Properties Based on Water Glass and Ambient Pressure Drying. Nanomaterials 2021, 11, 258. [Google Scholar] [CrossRef]
- Hung, W.-C.; Horng, R.S.; Shia, R.-E. Investigation of thermal insulation performance of glass/carbon fiber-reinforced silica aerogel composites. J. Sol-Gel Sci. Technol. 2021, 97, 414–421. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Begum, H.; Schoenwald, S.; Horoshenkov, K.V.; Malfait, W.J. A review on silica aerogel-based materials for acoustic applications. J. Non-Cryst. Solids 2021, 562, 120770–120786. [Google Scholar] [CrossRef]
- Rasyid, M.A.; Salim, M.; Akil, H.; Karger-Kocsis, J.; Ishak, Z.M. Non-woven flax fibre reinforced acrylic based polyester composites: The effect of sodium silicate on mechanical, flammability and acoustic properties. Express Polym. Lett. 2019, 13, 553–564. [Google Scholar] [CrossRef]
- Lin, J.; Li, G.; Liu, W.; Qiu, R.; Wei, H.; Zong, K.; Cai, X. A review of recent progress on the silica aerogel monoliths: Synthesis, reinforcement, and applications. J. Mater. Sci. 2021, 56, 10812–10833. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Boday, D.J.; Keng, P.Y.; Muriithi, B.; Pyun, J.; Loy, D.A. Mechanically reinforced silica aerogel nanocomposites via surface initiated atom transfer radical polymerizations. J. Mater. Chem. 2010, 20, 6863–6865. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.; Tousley, M.E.; Shonkwiler, B.; McCorkle, L.; Scheiman, D.A.; Palczer, A. Tailoring elastic properties of silica aerogels cross-linked with polystyrene. ACS Appl. Mater. Interfaces 2009, 1, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dass, A.; Rawashdeh, A.-M.M.; Thomas, J.; Counsil, J.A.; Sotiriou-Leventis, C.; Fabrizio, E.F.; Ilhan, F.; Vassilaras, P.; Scheiman, D.A.; et al. Isocyanate-crosslinked silica aerogel monoliths: Preparation and characterization. J. Non-Cryst. Solids 2004, 350, 152–164. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Weber, A.S.; Hindi, A.; Naumenko, M.; McCorkle, L.; Quade, D.; Vivod, S.L.; Gould, G.L.; White, S.; Deshpande, K. Structure-Property Relationships in Porous 3D Nanostructures: Epoxy-Cross-Linked Silica Aerogels Produced Using Ethanol as the Solvent. ACS Appl. Mater. Interfaces 2009, 1, 894–906. [Google Scholar] [CrossRef]
- Sai, H.; Wang, M.; Miao, C.; Song, Q.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L.; Hao, Y. Robust Silica-Bacterial Cellulose Composite Aerogel Fibers for Thermal Insulation Textile. Gels 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Sai, H.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Zhao, C.; Li, Z.; Li, F. Flexible aerogels based on an interpenetrating network of bacterial cellulose and silica by a non-supercritical drying process. J. Mater. Chem. A 2013, 1, 7963–7970. [Google Scholar] [CrossRef]
- Cai, J.; Liu, S.L.; Feng, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L.N. Cellulose-Silica Nanocomposite Aerogels by In Situ Formation of Silica in Cellulose Gel. Angew. Chem. Int. Ed. 2012, 51, 2076–2079. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Zhang, Z.; Sebe, G.; Wu, R.; Virtudazo, R.V.R.; Tingaut, P.; Koebel, M.M. Multiscale Assembly of Superinsulating Silica Aerogels within Silylated Nanocellulosic Scaffolds: Improved Mechanical Properties Promoted by Nanoscale Chemical Compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Sai, H.; Zhang, J.; Jin, Z.; Fu, R.; Wang, M.; Wang, Y.; Wang, Y.; Ma, L. Robust Silica-Cellulose Composite Aerogels with a Nanoscale Interpenetrating Network Structure Prepared Using a Streamlined Process. Polymers 2020, 12, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoda, S.; Takeshita, S.; Ono, T.; Tada, R.; Ota, H. Development of a New Silica Aerogel-Polypropylene Foam Composite as a Highly Flexible Thermal Insulation Material. Front. Mater. 2021, 8, 674846–674854. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 17187–17198. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.J.; Wang, S.Q.; He, C.X.; Lu, Z.X.; Huang, J.D.; Chen, Z.L. Facilitated fabrication of high strength silica aerogels using cellulose nanofibrils as scaffold. Carbohydr. Polym. 2016, 147, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Zhang, C. Versatile fabrication of anisotropic and superhydrophobic aerogels for highly selective oil absorption. Carbon 2019, 155, 16–24. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef]
- Gurav, J.L.; Rao, A.V.; Nadargi, D.Y.; Park, H.-H. Ambient pressure dried TEOS-based Silica aerogels: Good absorbents of organic liquids. J. Mater. Sci. 2010, 45, 503–510. [Google Scholar] [CrossRef]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Gao, R.; Xiao, S.; Gan, W.; Liu, Q.; Amer, H.; Rosenau, T.; Li, J.; Lu, Y. Mussel adhesive-inspired design of superhydrophobic nanofibrillated cellulose aerogels for oil/water separation. ACS Sustain. Chem. Eng. 2018, 6, 9047–9055. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, X.; Li, C.; You, B.; Sun, G. Co-gel strategy for preparing hierarchically porous silica/polyimide nanocomposite aerogel with thermal insulation and flame retardancy. J. Mater. Chem. A 2020, 8, 9701–9712. [Google Scholar] [CrossRef]
- Luo, Y.; Jiang, Y.; Feng, J.; Feng, J. Synthesis of white cement bonded porous fumed silica-based composite for thermal insulation with low thermal conductivity via a facile cast-in-place approach. Constr. Build. Mater. 2019, 206, 620–629. [Google Scholar] [CrossRef]
- Du, H.; Wang, S.; Xing, Y.; Li, X.; Pan, M.; Qi, W.; Ma, C. The dual effect of zirconia fiber on the insulation and mechanical performance of the fumed silica-based thermal insulation material. Ceram. Int. 2022, 48, 6657–6662. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, Y.; Kong, Y.; Ren, J.; Jiang, X.; Yan, W.; Li, M.; Tang, J.; Liu, X.; Shen, X. Thermal and Mechanical Performances of the Superflexible, Hydrophobic, Silica-Based Aerogel for Thermal Insulation at Ultralow Temperature. ACS Appl. Mater. Interfaces 2021, 13, 21286–21298. [Google Scholar] [CrossRef]
- Wernery, J.; Mancebo, F.; Malfait, W.J.; O’Connor, M.; Jelle, B.P. The economics of thermal superinsulation in buildings. Energy Build. 2021, 253, 111506–111523. [Google Scholar] [CrossRef]
- Ye, S.; Wang, B.; Shi, Y.; Wang, B.; Zhang, Y.; Feng, Y.; Han, W.; Liu, C.; Shen, C. Superhydrophobic and superelastic thermoplastic polyurethane/multiwalled carbon nanotubes porous monolith for durable oil/water separation. Compos. Commun. 2020, 21, 100378–100385. [Google Scholar] [CrossRef]
- Yi, L.; Yang, J.; Fang, X.; Xia, Y.; Zhao, L.; Wu, H.; Guo, S. Facile fabrication of wood-inspired aerogel from chitosan for efficient removal of oil from Water. J. Hazard Mater. 2020, 385, 121507–121516. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]







| Sample | Bulk Density (g/cm3) | Porosity a (%) | SBET (m2/g) | Average Pore Size b (nm) | Pore Volume c (cm3/g) | Compression Modulus (MPa) | Thermal Conductivity (mW m−1 K−1) |
|---|---|---|---|---|---|---|---|
| CA-1 | 0.079 | 96.0 | 272.4 | 10.5 | 0.78 | 0.68 | 28.9 |
| CA-2 | 0.107 | 94.8 | 304.8 | 10.4 | 0.86 | 2.90 | 30.5 |
| CA-3 | 0.123 | 94.0 | 375.3 | 11.1 | 1.21 | 5.05 | 32.3 |
| CA-4 | 0.128 | 93.8 | 420.5 | 11.8 | 1.32 | 6.23 | 34.6 |
| AA-1 | 0.029 | 98.4 | 269.1 | 13.6 | 0.88 | 0.21 | 32.2 |
| AA-2 | 0.021 | 98.8 | 239.0 | 14.1 | 0.76 | 0.13 | 30.4 |
| AA-3 | 0.019 | 98.9 | 227.8 | 14.2 | 0.65 | 0.08 | 28.7 |
| AA-4 | 0.018 | 99.0 | 219.9 | 14.3 | 0.60 | 0.04 | 26.3 |
| SA-1 | 0.062 | 97.0 | 742.3 | 13.8 | 2.55 | d | e |
| SA-2 | 0.089 | 95.8 | 754.8 | 14.8 | 2.61 | d | e |
| SA-3 | 0.107 | 94.9 | 767.0 | 14.9 | 3.06 | d | e |
| SA-4 | 0.116 | 94.5 | 839.0 | 16.7 | 3.25 | d | e |
| Materials | Density (g/cm3) | SBET (m2/g) | Pore Volume (cm3/g) | Compression Modulus (MPa) | Thermal Conductivity (mW m−1 K−1) | Ref. |
|---|---|---|---|---|---|---|
| AG–SiO2 composite aerogel | 0.079~0.128 | 272.4~420.5 | 0.73~1.09 | 0.68~6.23 | 28.9~34.6 | This work |
| polyurethane foam | not reported | not reported | not reported | not reported | 20~50 | [18] |
| mineral wool | not reported | not reported | not reported | not reported | 35~80 | [18] |
| SiO2/PI nanocomposite aerogel | not reported | not reported | not reported | 1.9 | 31.1~41.6 | [51] |
| SiO2–SSNF aerogel | 0.085~0.093 | not reported | not reported | 30~70 | 25~29 | [9] |
| silica nanotube aerogels | 0.025 | 327~427 | 0.99~1.15 | 0.3~1.9 | 30.2~32.6 | [23] |
| fumed silica insulation | 0.5~1.2 | not reported | not reported | 0.15 | 33 | [52] |
| ZrO2 fiber/GF and fumed SiO2/Al2O3 | 0.733~0.761 | not reported | 0.04~0.05 | 0.02~0.18 | 50~77 | [53] |
| hydrophobic silica-based aerogel | 0.047~0.077 | 28.4~337.0 | 0.059~0.267 | 0.2 | 24 | [54] |
| silica aerogels blanket/ board | 0.08~0.2 | 600~800 | not reported | not reported | ≥15 | [55] |
| Abbreviations | Full Names | Abbreviations | Full Names |
|---|---|---|---|
| AG | agarose | BJH | Barrett–Joyner–Halenda |
| SiO2 | silica | TGA | thermogravimetric analysis |
| CAs | composite aerogels | SEM | scanning electron microscopy |
| AAs | agarose aerogels | ATR-FTIR | attenuated total reflection fourier transform infrared |
| SAs | silica aerogels | EDS | energy-dispersive X-ray spectra |
| SSNF | SiO2/SnO2 nanofibers | SCF | supercritical CO2 fluid |
| GF | glass fiber | TEOS | tetraethyl orthosilicate |
| PI | polyimide | IPN | Interpenetrating network |
| BET | Brunner−Emmet−Teller | PET | polyethylene terephthalate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Jiang, P.; Xiao, R.; Fu, R.; Liu, Y.; Ji, C.; Song, Q.; Miao, C.; Yu, H.; Gu, J.; et al. Robust Silica–Agarose Composite Aerogels with Interpenetrating Network Structure by In Situ Sol–Gel Process. Gels 2022, 8, 303. https://doi.org/10.3390/gels8050303
Yang X, Jiang P, Xiao R, Fu R, Liu Y, Ji C, Song Q, Miao C, Yu H, Gu J, et al. Robust Silica–Agarose Composite Aerogels with Interpenetrating Network Structure by In Situ Sol–Gel Process. Gels. 2022; 8(5):303. https://doi.org/10.3390/gels8050303
Chicago/Turabian StyleYang, Xin, Pengjie Jiang, Rui Xiao, Rui Fu, Yinghui Liu, Chao Ji, Qiqi Song, Changqing Miao, Hanqing Yu, Jie Gu, and et al. 2022. "Robust Silica–Agarose Composite Aerogels with Interpenetrating Network Structure by In Situ Sol–Gel Process" Gels 8, no. 5: 303. https://doi.org/10.3390/gels8050303