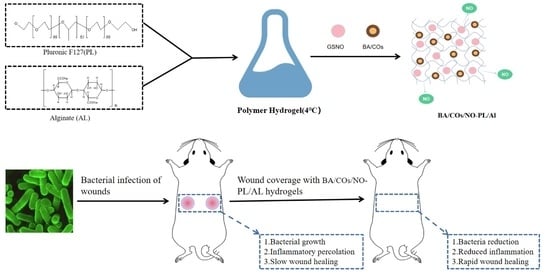
Dual-Delivery Temperature-Sensitive Hydrogel with Antimicrobial and Anti-Inflammatory Brevilin A and Nitric Oxide for Wound Healing in Bacterial Infection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Submicron Emulsion
2.2. BA/COs/NO-PL/AL Synthesis and Characterization of Hydrogels
2.3. BA/COs/NO-PL/AL Drug Dissolution
2.4. Cytotoxic Activity of Hydrogels against L929
2.5. In Vitro Anti-Inflammatory Activity
2.6. In Vitro Antibacterial Activity
2.7. Healing Effect of Hydrogel on MRPA-Infected Wounds
2.8. Wound Colony Load and Inflammatory Expression
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. BA Submicron Emulsion Preparation
4.3. GSNO Synthesis
4.4. Preparation of BA/COs/NO-PL/AL Gels
4.5. Particle Size, PDI, and Zeta Potential Measurements
4.6. Centrifugal Stability Coefficient Ke Value
4.7. Thermal Responsiveness of Hydrogel
4.8. Rheology of Hydrogels
4.9. Thermal Analysis of Hydrogels
4.10. Drug Dissolution
4.11. Cytotoxic Activity of Hydrogels against L929
4.12. RAW 264.7 Determination of Cell Viability
4.13. In Vitro Anti-Inflammatory Activity
4.14. In Vitro Antibacterial Activity
4.15. In Vivo Evaluation of Wound Healing
4.15.1. Healing Effect of Hydrogel on MRPA-Infected Wounds
4.15.2. Wound Colony Load
4.15.3. Detection of Inflammatory Biomarkers in Skin Wound Tissue
4.15.4. Organizational Assessment
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Mauro, T.M.; Dang, E.; Man, G.; Zhang, J.; Lee, D.; Wang, G.; Feingold, K.R.; Elias, P.M.; Man, M.Q. Epidermal Dysfunction Leads to an Age-Associated Increase in Levels of Serum Inflammatory Cytokines. J. Investig. Dermatol. 2017, 137, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kopecki, Z. Development of next-generation antimicrobial hydrogel dressing to combat burn wound infection. Biosci. Rep. 2021, 41, BSR20203404. [Google Scholar] [CrossRef]
- Menezes, R.; Vincent, R.; Osorno, L.; Hu, P.; Arinzeh, T.L. Biomaterials and tissue engineering approaches using glycosaminoglycans for tissue repair: Lessons learned from the native extracellular matrix. Acta Biomater. 2023, 163, 210–227. [Google Scholar] [CrossRef]
- Li, W.; Su, H.; Ma, Y.; Ren, H.; Feng, Z.; Wang, Y.; Qiu, Y.; Wang, H.; Wang, H.; Chen, Q.; et al. Multicargo-loaded inverse opal gelatin hydrogel microparticles for promoting bacteria-infected wound healing. Int. J. Biol. Macromol. 2024, 260, 129557. [Google Scholar] [CrossRef]
- Sen, R.K.; Prabhakar, P.; Mayandi, V.; Dwivedi, N.; Yadav, A.K.; Solanki, P.R.; Gupta, A.; Gowri, V.S.; Lakshminarayanan, R.; Verma, N.K.; et al. Metal mediated high performance antimicrobial hydrogel films for wound infection management: Zn, Cu, and Mg versus Ag and Au. Mater. Chem. Phys. 2023, 297, 127365. [Google Scholar] [CrossRef]
- Yan, J.; Ji, Y.; Huang, M.; Li, T.; Liu, Y.; Lu, S.; Liu, M. Nucleobase-Inspired Self-Adhesive and Inherently Antibacterial Hydrogel for Wound Dressing. ACS Mater. Lett. 2020, 2, 1375–1380. [Google Scholar] [CrossRef]
- Yang, L.; Feura, E.S.; Ahonen, M.J.R.; Schoenfisch, M.H. Nitric Oxide-Releasing Macromolecular Scaffolds for Antibacterial Applications. Adv. Healthc. Mater. 2018, 7, e1800155. [Google Scholar] [CrossRef]
- Zhang, S.; Guan, K.; Zhang, Y.; Zhang, J.; Fu, H.; Wu, T.; Ouyang, D.; Liu, C.; Wu, Q.; Chen, Z. A self-activated NO-releasing hydrogel depot for photothermal enhanced sterilization. Nano Res. 2023, 16, 5346–5356. [Google Scholar] [CrossRef]
- Wang, D.C.; Clark, J.R.; Lee, R.; Nelson, A.H.; Maresso, A.W.; Acharya, G.; Shin, C.S. Development of Antimicrobial Nitric Oxide-Releasing Fibers. Pharmaceutics 2021, 13, 1445. [Google Scholar] [CrossRef]
- Man, M.Q.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M. Regulatory role of nitric oxide in cutaneous inflammation. Inflammation 2022, 45, 949–964. [Google Scholar] [CrossRef]
- Seabra, A.B.; Pieretti, J.C.; de Melo Santana, B.; Horue, M.; Tortella, G.R.; Castro, G.R. Pharmacological applications of nitric oxide-releasing biomaterials in human skin. Int. J. Pharm. 2023, 630, 122465. [Google Scholar] [CrossRef]
- Xu, X.; Zeng, Y.; Chen, Z.; Yu, Y.; Wang, H.; Lu, X.; Zhao, J.; Wang, S. Chitosan-based multifunctional hydrogel for sequential wound inflammation elimination, infection inhibition, and wound healing. Int. J. Biol. Macromol. 2023, 235, 123847. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Chen, X.; Xu, C.; Guo, J.; Deng, M.; Qu, X.; Huang, P.; Feng, Z.; Zhang, J. Versatile Hydrogel Dressing with Skin Adaptiveness and Mild Photothermal Antibacterial Activity for Methicillin-Resistant Staphylococcus Aureus-Infected Dynamic Wound Healing. Adv. Sci. 2023, 10, e2206585. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sharma, A.K.; Gupta, V.; Yashavarddhan, M.H. Pharmacological control of inflammation in wound healing. J. Tissue Viability 2019, 28, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2021, 18, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Swanson, T.; Ousey, K.; Haesler, E.; Bjarnsholt, T.; Carville, K.; Idensohn, P.; Kalan, L.; Keast, D.H.; Larsen, D.; Percival, S.; et al. IWII Wound Infection in Clinical Practice consensus document: 2022 update. J. Wound Care 2022, 31, S10–S21. [Google Scholar] [CrossRef] [PubMed]
- Holzer-Geissler, J.C.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X.; Yuan, Y.; Hu, Q.; Tang, J.; Xie, C. Brevilin A Ameliorates Acute Lung Injury and Inflammation Through Inhibition of NF-κB Signaling via Targeting IKKα/β. Front. Pharmacol. 2022, 13, 911157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Yan, Y.M.; Li, S.Y.; He, D.H.; Xiong, S.; Wei, S.F.; Liu, W.; Hu, L.; Wang, Q.; Pan, H.F.; et al. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol. Sin. 2020, 41, 10–21. [Google Scholar] [CrossRef]
- Huang, S.S.; Chiu, C.S.; Lin, T.H.; Lee, M.M.; Lee, C.Y.; Chang, S.J.; Hou, W.C.; Huang, G.J.; Deng, J.S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef]
- Yen, L.J.; Yen, C.Y.; Li, C.L.; Liao, E.C.; Wang, K.C.; Shih, M.C.; Huang, H.S.; Chen, Y.C.; Lu, L.Y.; Yu, S.J. Brevilin A Ameliorates Imiquimod-Induced Psoriasis-like Dermatitis and Reduces Th17 Differentiation in Psoriasis Patients. J. Pers. Med. 2022, 12, 1888. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, F.; Wu, Q.; Li, S.; Ruan, G.; Yang, J.; Yu, C.; Jiang, N.; Xiao, Y.; Liu, Y. Brevilin A enhances innate immunity and the resistance of oxidative stress in Caenorhabditis elegans via p38 MAPK pathway. Int. Immunopharmacol. 2022, 113, 109385. [Google Scholar] [CrossRef]
- Güngör, S.; Kahraman, E. Nanocarriers Mediated Cutaneous Drug Delivery. Eur. J. Pharm. Sci. 2021, 158, 105638. [Google Scholar] [CrossRef]
- Yong, Z.; Xingqi, W.; Jie, H.; Rongfeng, H.; Xiaoqin, C. Formulation, production, in vitro release and in vivo pharmacokinetics of cinnamaldehyde sub-micron emulsions. Pharm. Dev. Technol. 2020, 25, 676–685. [Google Scholar] [CrossRef]
- Jangdey, M.S.; Gupta, A.; Saraf, S. Fabrication, in-vitro characterization, and enhanced in-vivo evaluation of carbopol-based nanoemulsion gel of apigenin for UV-induced skin carcinoma. Drug Deliv. 2017, 24, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Marti-Mestres, G.; Ramos, J.; Maillols, H. LC analysis of benzophenone-3: II application to determination of ‘in vitro’ and ‘in vivo’ skin penetration from solvents, coarse and submicron emulsions. J. Pharm. Biomed. Anal. 2000, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Marlowe, Z.T.; Cavet, M.E.; Coffey, M.J. Dose Uniformity of Loteprednol Etabonate (Submicron) Ophthalmic Gel 0.38% Compared with Prednisolone Acetate Ophthalmic Suspension 1. Ophthalmol. Ther. 2022, 11, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, A.; Du, Q.; Zhu, W.; Liu, H.; Naeem, A.; Guan, Y.; Chen, L.; Ming, L. Bioactive substances and therapeutic potential of camellia oil: An overview. Food Biosci. 2022, 49, 101855. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, S.Y.; Lee, W.T.; Yen, G.C. Immunomodulatory effect of camellia oil (Camellia oleifera Abel.) on CD19+B cells enrichment and IL-10 production in BALB/c mice. J. Funct. Foods 2022, 88, 104863. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Liang, R.; Liu, C.; Chen, M.; Chen, J. Synergistic Anti-Inflammatory Effects of Lipophilic Grape Seed Proanthocyanidin and Camellia Oil Combination in LPS-Stimulated RAW2647 Cells. Antioxidants 2022, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Lehri, D.; Kumari, N.; Singh, R.P. Ultrasound-assisted production and characterization of rice bran lecithin-based nanoemulsions. J. Dispers. Sci. Technol. 2020, 42, 1368–1375. [Google Scholar] [CrossRef]
- Hlaing, S.P.; Kim, J.; Lee, J.; Hasan, N.; Cao, J.; Naeem, M.; Lee, E.H.; Shin, J.H.; Jung, Y.; Lee, B.L.; et al. S-Nitrosoglutathione loaded poly(lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphylococcus aureus-infected wounds. Eur. J. Pharm. Biopharm. 2018, 132, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Su, M.; Hasan, N.; Lee, J.; Kwak, D.; Kim, D.Y.; Kim, K.; Lee, E.H.; Jung, J.H.; Yoo, J.-W. Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics 2020, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jin, L.; Liu, Q.; Zhao, K.; Lin, L.; Zheng, J.; Li, C.; Chen, B.; Shen, Y. Recent advances in the extraction, composition analysis and bioactivity of Camellia (Camellia oleifera Abel.) oil. Trends Food Sci. Technol. 2024, 143, 104211. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, N.; Pan, S.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Roburic Acid Suppresses NO and IL-6 Production via Targeting NF-κB and MAPK Pathway in RAW264.7 Cells. Inflammation 2017, 40, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, G.; Hong, W.; Zhang, Y.; Xu, B.; Song, G.; Liu, T.; Hong, C.; Ruan, L. Rapid gelation of oxidized hyaluronic acid and succinyl chitosan for integration with insulin-loaded micelles and epidermal growth factor on diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111273. [Google Scholar] [CrossRef]
- Zeng, Q.; Qian, Y.; Huang, Y.; Ding, F.; Qi, X.; Shen, J. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioact. Mater. 2021, 6, 2647–2657. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Yang, C.; Zhang, H.; Fareed, M.S.; He, Y.; Su, J.; Wang, P.; Shen, Z.; Yan, W.; et al. A carrier-free, dual-functional hydrogel constructed of antimicrobial peptide Jelleine-1 and 8Br-cAMP for MRSA infected diabetic wound healing. Acta Biomater. 2022, 151, 223–234. [Google Scholar] [CrossRef]
- Ramos-Bell, S.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Ragazzo-Sánchez, J.A. Characterization of submicron emulsion processed by ultrasound homogenization to protect a bioactive extract from sea grape (Coccoloba uvifera L.). Food Sci. Biotechnol. 2020, 29, 1365–1372. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Sun, R.; Li, T.; Xia, N.; Xia, Q. Antioxidant activity, in vitro digestibility and stability of flaxseed oil and quercetin co-loaded submicron emulsions. Eur. J. Lipid Sci. Technol. 2017, 120, 1700441. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L.; Kan, C.W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef] [PubMed]
- Cabral, F.V.; Santana, B.D.M.; Lange, C.N.; Batista, B.L.; Seabra, A.B.; Ribeiro, M.S. Pluronic F-127 hydrogels containing copper oxide nanoparticles and a nitric oxide donor to treat skin cancer. Pharmaceutics 2023, 15, 1971. [Google Scholar] [CrossRef]
- Wróblewska, M.; Szymańska, E.; Winnicka, K. The Influence of Tea Tree Oil on Antifungal Activity and Pharmaceutical Characteristics of Pluronic® F-127 Gel Formulations with Ketoconazole. Int. J. Mol. Sci. 2021, 22, 11326. [Google Scholar] [CrossRef]
- Ho, C.L.; Li, L.H.; Weng, Y.C.; Hua, K.F.; Ju, T.C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement. Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Son, K.N.; Shah, D.; Ali, M.; Balasubramaniam, A.; Shukla, D.; Aakalu, V.K. Histatin-1 Attenuates LPS-Induced Inflammatory Signaling in RAW264.7 Macrophages. Int. J. Mol. Sci. 2021, 22, 7856. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, Y.; Zhou, F.; Hu, Y.; Zhao, J.; Liu, Z.; Zhai, Q.; Qi, S.; Zhang, Z.; Chen, L. Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioeng. Transl. Med. 2023, 8, e10373. [Google Scholar] [CrossRef]










| Name | Size (nm) | PDI | Zeta Potential (mV) | Ke |
|---|---|---|---|---|
| COs | 237.82 ± 0.54 | 0.183 ± 0.04 | −38.67 ± 0.19 | 0.13 ± 0.09 |
| BA/COs | 239.18 ± 1.47 | 0.183 ± 0.04 | −38.25 ± 0.27 | 0.22 ± 0.09 |
| Hydrogels | Tonset (°C) | Tpeak (°C) | Tendset (°C) | ΔH (mJ) | Tgel (°C) | Loading (%) |
|---|---|---|---|---|---|---|
| PL/AL | 17.45 | 19.82 | 25.55 | −83.21 | 24.7 | N.D. |
| NO-PL/AL | 15.07 | 17.87 | 21.97 | −84.54 | 22.4 | 0.172 ± 0.01% * |
| COs/NO-PL/AL | 13.45 | 14.72 | 20.58 | −87.49 | 21.9 | 0.169 ± 0.01% * |
| BA/COs/NO-PL/AL | 13.52 | 14.65 | 20.52 | −77.78 | 21.8 | 0.171 ± 0.02% * 0.009 ± 0.00% # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, L.; Pan, C.; Ran, X.; Wen, Y.; Lang, R.; Peng, M.; Cao, J.; Yang, J. Dual-Delivery Temperature-Sensitive Hydrogel with Antimicrobial and Anti-Inflammatory Brevilin A and Nitric Oxide for Wound Healing in Bacterial Infection. Gels 2024, 10, 219. https://doi.org/10.3390/gels10040219
Ruan L, Pan C, Ran X, Wen Y, Lang R, Peng M, Cao J, Yang J. Dual-Delivery Temperature-Sensitive Hydrogel with Antimicrobial and Anti-Inflammatory Brevilin A and Nitric Oxide for Wound Healing in Bacterial Infection. Gels. 2024; 10(4):219. https://doi.org/10.3390/gels10040219
Chicago/Turabian StyleRuan, Linghui, Chengfeng Pan, Xianting Ran, Yonglan Wen, Rui Lang, Mei Peng, Jiafu Cao, and Juan Yang. 2024. "Dual-Delivery Temperature-Sensitive Hydrogel with Antimicrobial and Anti-Inflammatory Brevilin A and Nitric Oxide for Wound Healing in Bacterial Infection" Gels 10, no. 4: 219. https://doi.org/10.3390/gels10040219