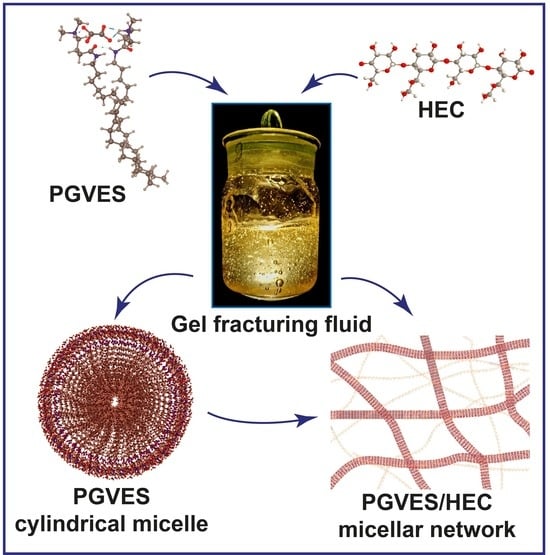
Experimental Investigation of Hydraulic Fracturing Fluid Based on Pseudo Gemini Surfactant with Polysaccharide Addition
Abstract
:1. Introduction
- Viscosity values are lower compared with cross-linked guar gel, which promotes the formation of long and branched fractures;
- High viscoelasticity in fresh and mineralized water, due to which the fracturing has the necessary sand-carrying ability;
- The use of surfactants increases the oil recovery factor;
- Hydrophobization of terrigenous rock and clays, as a result of which the degree of clays’ swelling is reduced, and water blockages do not form after hydraulic fracturing;
- Viscosity reduction when mixed with formation fluids, resulting in complete restoration of rock permeability after hydraulic fracturing (pure fracturing).
2. Results and Discussion
2.1. Effective Viscosity Study
2.2. Oscillation Experiments
2.3. Rheological Studies at Elevated Temperatures
2.4. Determination of Proppant Particle Settling Velocity
2.5. Fluid-Loss
3. Conclusions
- Rotational and oscillatory studies showed a significant increase in viscosity and viscoelastic properties with the introduction of HEC into the compositions. However, with increasing temperature, these properties degrade more strongly in gels with polymer. For further experiments at elevated temperatures, we recommend the use of cationic or anionic polymers that will interact with PGVES by electrostatic mechanism, this will give better thermostability of the gel. The temperature values used in this work correspond to the field conditions in the Urals and Volga regions of the Russian Federation, and the developed compositions are recommended for these locations;
- In the case of gels with the addition of HEC, the storage modulus on the plateau remains consistently large, which indicates a high density of the micellar network;
- Subsequent studies on the settling velocity of proppant show that the settling velocity of proppant at elevated temperatures, especially fine fractions, is lower for a gel with an injected polymer. It can be concluded that the density of the micelle network has a strong influence on this parameter. Based on this experiment, we recommend the use of coarse proppant fractions in gels with polymer addition at temperatures up to 50 °C. However, good sand-carrying capacity can also be achieved at higher temperatures due to turbulence flow;
- The addition of HEC helps to reduce the degree of fluid loss, which was revealed during experiments on a fluid-loss cell. Formation damage experiments are recommended as further studies.
4. Materials and Methods
4.1. Materials
4.2. Preparation of Fracturing Gel
4.3. Research Methods
4.3.1. Rotational Viscometry and Oscillatory Rheology
4.3.2. Determination of Proppant Particle Settling Velocity
4.3.3. Experiments on a Fluid-Loss Cell
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Muntasheri, G.A. A Critical Review of Hydraulic-Fracturing Fluids for Moderate- to Ultralow- Permeability Formations over the Last Decade. SPE Prod. Oper. 2014, 29, 243–260. [Google Scholar] [CrossRef]
- Chen, B.; Barboza, B.R.; Sun, Y.; Bai, J.; Thomas, H.R.; Dutko, M.; Cottrell, M.; Li, C. A Review of Hydraulic Fracturing Simulation. Arch. Comput. Methods Eng. 2021, 29, 1–58. [Google Scholar] [CrossRef]
- Osiptsov, A.A. Fluid Mechanics of Hydraulic Fracturing: A Review. J. Pet. Sci. Eng. 2017, 156, 513–535. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A Review of Fracturing Fluid Systems Used for Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131, 40735. [Google Scholar] [CrossRef]
- Hurnaus, T.; Plank, J. Behavior of Titania Nanoparticles in Cross-Linking Hydroxypropyl Guar Used in Hydraulic Fracturing Fluids for Oil Recovery. Energy Fuels 2015, 29, 3601–3608. [Google Scholar] [CrossRef]
- Trabelsi, S.; Kakadjian, S. Comparative Study Between Guar and Carboxymethylcellulose Used as Gelling Systems in Hydraulic Fracturing Application. SPE Prod. Oper. Symp. Proc. 2013, 183–202. [Google Scholar] [CrossRef]
- Ihejirika, B.; Dosunmu, A.; Eme, C. Performance Evaluation of Guar Gum as a Carrier Fluid for Hydraulic Fracturing. In Proceedings of the Society of Petroleum Engineers—SPE Nigeria Annual International Conference and Exhibition, NAICE 2015, Lagos, Nigeria, 4–6 August 2015. [Google Scholar] [CrossRef]
- Coveney, P.V.; De Silva, H.; Gomtsyan, A.; Whiting, A.; Boek, E.S. Novel Approaches to Cross-Linking High Molecular Weight Polysaccharides: Application to Guar-Based Hydraulic Fracturing Fluids. Mol. Simul. 2006, 25, 265–299. [Google Scholar] [CrossRef]
- Kesavan, S.; Prud’homme, R.K. Rheology of Guar and HPG Cross-Linked by Borate. Macromolecules 1992, 25, 2026–2032. [Google Scholar] [CrossRef]
- Wang, S.; Tang, H.; Guo, J.; Wang, K. Effect of PH on the Rheological Properties of Borate Crosslinked Hydroxypropyl Guar Gum Hydrogel and Hydroxypropyl Guar Gum. Carbohydr. Polym. 2016, 147, 455–463. [Google Scholar] [CrossRef]
- Hurnaus, T.; Plank, J. Crosslinking of Guar and HPG Based Fracturing Fluids Using ZrO2 Nanoparticles. Proc. SPE Int. Symp. Oilfield Chem. 2015, 2, 988–1002. [Google Scholar] [CrossRef]
- Nimerick, K.H.; Temple, H.L.; Card, R.J. New PH-Buffered Low Polymer Borate Crosslinked Fluids for Hydraulic Fracturing. SPE J. 1997, 2, 150–156. [Google Scholar] [CrossRef]
- Guo, T.; Gong, F.; Lin, X.; Lin, Q.; Wang, X. Experimental Investigation on Damage Mechanism of Guar Gum Fracturing Fluid to Low-Permeability Reservoir Based on Nuclear Magnetic Resonance. J. Energy Resour. Technol. Trans. ASME 2018, 140, 072906. [Google Scholar] [CrossRef]
- Chieng, Z.H.; Mohyaldinn, M.E.; Hassan, A.M.; Bruining, H. Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid. Polymers 2020, 12, 1470. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.N.C.; Santanna, V.C.; Neto, A.A.D.; Neto, E.L.B. Application of Surfactants for Obtaining Hydraulic Fracturing Gel. Pet. Sci. Technol. 2003, 21, 1145–1157. [Google Scholar] [CrossRef]
- Das, A.; Chauhan, G.; Verma, A.; Kalita, P.; Ojha, K. Rheological and Breaking Studies of a Novel Single-Phase Surfactant-Polymeric Gel System for Hydraulic Fracturing Application. J. Pet. Sci. Eng. 2018, 167, 559–567. [Google Scholar] [CrossRef]
- Samuel, M.; Polson, D.; Graham, D.; Kordziel, W.; Waite, T.; Waters, G.; Vinod, P.S.; Fu, D.; Downey, R. Viscoelastic Surfactant Fracturing Fluids: Applications in Low Permeability Reservoirs. In Proceedings of the SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition, Denver, Colorado, 12–15 March 2000. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Negash, B.M.; Rahman, M.M.; Haroun, M.; Al-Shami, T.M. Perspective Review of Polymers as Additives in Water-Based Fracturing Fluids. ACS Omega 2022, 7, 7431–7443. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Guo, J.; Gou, X.; Jiang, Z.; Pan, B. Reduced Adsorption of Polyacrylamide-Based Fracturing Fluid on Shale Rock Using Urea. Energy Sci. Eng. 2018, 6, 749–759. [Google Scholar] [CrossRef]
- Telin, A.; Lenchenkova, L.; Yakubov, R.; Poteshkina, K.; Krisanova, P.; Filatov, A.; Stefantsev, A. Application of Hydrogels and Hydrocarbon-Based Gels in Oil Production Processes and Well Drilling. Gels 2023, 9, 609. [Google Scholar] [CrossRef]
- Wattebled, L.; Laschewsky, A. Effects of Organic Salt Additives on the Behavior of Dimeric (“gemini”) Surfactants in Aqueous Solution. Langmuir 2007, 23, 10044–10052. [Google Scholar] [CrossRef]
- Qiao, Y.; Lin, Y.; Wang, Y.; Li, Z.; Huang, J. Metal-Driven Viscoelastic Wormlike Micelle in Anionic/Zwitterionic Surfactant Systems and Template-Directed Synthesis of Dendritic Silver Nanostructures. Langmuir 2011, 27, 1718–1723. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, J.; Mao, J.; Yang, X.; Zhang, H.; Zhang, W. High Performance Clean Fracturing Fluid Using a New Tri-Cationic Surfactant. Polymers 2018, 10, 535. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Ahmed, H. Novel Cationic Gemini Surfactants as Corrosion Inhibitors for Carbon Steel Pipelines. Corros. Sci. 2010, 52, 2897–2904. [Google Scholar] [CrossRef]
- Hanson, M.E.; Shaffer, R.J.; Anderson, G.D. Effects of Various Parameters on Hydraulic Fracturing Geometry. Soc. Pet. Eng. J. 1981, 21, 435–443. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhang, Z.; Yang, B.; Zhang, Y.; Zhao, J. Study of a Novel Gemini Viscoelastic Surfactant with High Performance in Clean Fracturing Fluid Application. Polymers 2018, 10, 1215. [Google Scholar] [CrossRef]
- Li, Z.; Kang, W.; Zhao, Y.; Yang, H.; Li, M.; Kang, X.; Zhu, T.; Zhou, B.; Sarsenbekuly, B.; Aidarova, S. Organic Acid-Enhanced Viscoelastic Surfactant and Its Application in Fracturing Fluids. Energy Fuels 2021, 35, 3130–3139. [Google Scholar] [CrossRef]
- Wang, P.; Kang, W.; Yang, H.; Yin, X.; Zhao, Y.; Zhu, Z.; Zhang, X. PH-Responsive Wormlike Micelles Based on Microstructural Transition in a C22-Tailed Cationic Surfactant-Aromatic Dibasic Acid System. RSC Adv. 2017, 7, 37699–37705. [Google Scholar] [CrossRef]
- Kang, W.; Mushi, S.J.; Yang, H.; Wang, P.; Hou, X. Development of Smart Viscoelastic Surfactants and Its Applications in Fracturing Fluid: A Review. J. Pet. Sci. Eng. 2020, 190, 107107. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y. PH-Switchable Wormlike Micelles. Chem. Commun. 2010, 46, 9028–9030. [Google Scholar] [CrossRef]
- Yin, T.; Liu, H.; Wen, X.; Wang, W.; Wang, C.; Yan, Z.; Yang, Z. PH-Responsive Clean Fracturing Fluid Based on Pseudo-Trimeric Surfactants. Colloid. Polym. Sci. 2023, 301, 189–197. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Hou, X.; Wang, F.; Zhao, H.; Zhou, B.; Zhang, H.; Yang, H.; Kang, W. Effects of Sodium Chloride on Rheological Behaviour of the Gemini-like Surfactants. Soft Matter 2020, 16, 4024–4031. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, H.; Zhang, J.; Guo, Y.; Wei, B.; Wen, Y. Pseudo-Interpenetrating Network Viscoelastic Surfactant Fracturing Fluid Formed by Surface-Modified Cellulose Nanofibril and Wormlike Micelles. J. Pet. Sci. Eng. 2022, 208, 109608. [Google Scholar] [CrossRef]
- Cui, W.; Zou, H.; Wang, C.; Yang, J.; Yan, J. Supramolecular & Oil Displacement Fluid for Hydraulic Fracturing. In Proceedings of the Society of Petroleum Engineers—SPE Asia Pacific Oil and Gas Conference and Exhibition 2018, APOGCE 2018, Brisbane, Australia, 23–25 October 2018. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Zhou, C.; Cui, W.; Guan, B.; Qiu, X.; Liu, P.; Ming, H.; Qin, W.; Ji, S. Supramolecular Viscoelastic Surfactant Fluid for Hydraulic Fracturing. In Proceedings of the Society of Petroleum Engineers—SPE North Africa Technical Conference and Exhibition 2015, NATC 2015, Cairo, Egypt, 14–16 September 2015; pp. 516–524. [Google Scholar] [CrossRef]
- Shashkina, J.A.; Philippova, O.E.; Zaroslov, Y.D.; Khokhlov, A.R.; Pryakhina, T.A.; Blagodatskikh, I.V. Rheology of Viscoelastic Solutions of Cationic Surfactant. Effect of Added Associating Polymer. Langmuir 2005, 21, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qiu, G.; Long, X.; Wang, T.; Zhang, X.; Liang, L.; Bai, J.; Li, Z.; Qiu, L.; Yang, X.; et al. Hooked Gemini Viscoelastic Surfactant Based on Linolenic Oil for Oil Recovery and Its Various Gel-Breaking Mechanisms. J. Pet. Sci. Eng. 2021, 204, 108717. [Google Scholar] [CrossRef]
- Sun, C.; Boluk, Y. Rheological Behavior and Particle Suspension Capability of Guar Gum: Sodium Tetraborate Decahydrate Gels Containing Cellulose Nanofibrils. Cellulose 2016, 23, 3013–3022. [Google Scholar] [CrossRef]
- Anachkov, S.E.; Georgieva, G.S.; Abezgauz, L.; Danino, D.; Kralchevsky, P.A. Viscosity Peak Due to Shape Transition from Wormlike to Disklike Micelles: Effect of Dodecanoic Acid. Langmuir 2018, 34, 4897–4907. [Google Scholar] [CrossRef]
- Georgieva, G.S.; Anachkov, S.E.; Lieberwirth, I.; Koynov, K.; Kralchevsky, P.A. Synergistic Growth of Giant Wormlike Micelles in Ternary Mixed Surfactant Solutions: Effect of Octanoic Acid. Langmuir 2016, 32, 12885–12893. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Meng, X.; Wang, Q.; Han, X. Wormlike Micelles with a Unique Ladder Shape Formed by a C22-Tailed Zwitterionic Surfactant Bearing a Bulky Piperazine Group. Soft Matter 2019, 15, 7644–7653. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Z.; Song, Z.; Bai, J.; Zhang, Y.; Luo, J.Q.; Du, Y.; Jiang, Q. Self-Assembly Properties of Ultra-Long-Chain Gemini Surfactant with High Performance in a Fracturing Fluid Application. J. Appl. Polym. Sci. 2017, 134, 108717. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Yang, X.; Zhang, H.; Zhao, J.; Tian, J.; Lin, C.; Mao, J. Development of a Sulfonic Gemini Zwitterionic Viscoelastic Surfactant with High Salt Tolerance for Seawater-Based Clean Fracturing Fluid. Chem. Eng. Sci. 2019, 207, 688–701. [Google Scholar] [CrossRef]
- Kuryashov, D.A.; Philippova, O.E.; Molchanov, V.S.; Bashkirtseva, N.Y.; Diyarov, I.N. Temperature Effect on the Viscoelastic Properties of Solutions of Cylindrical Mixed Micelles of Zwitterionic and Anionic Surfactants. Colloid Journal 2010, 72, 230–235. [Google Scholar] [CrossRef]
- Agrawal, N.R.; Yue, X.; Feng, Y.; Raghavan, S.R. Wormlike Micelles of a Cationic Surfactant in Polar Organic Solvents: Extending Surfactant Self-Assembly to New Systems and Subzero Temperatures. Langmuir 2019, 35, 12782–12791. [Google Scholar] [CrossRef] [PubMed]
- ISO, 13503–4; Completion Fluids and Materials Part 4: Procedure for Measuring Stimulation and Gravel-Pack fluid Leakoff Under Static Conditions. ISO: Geneva, Switzerland, 2006.












| HEC Concentration (%) | (Pa) | (s) | (nm) | (Pa) |
|---|---|---|---|---|
| 0 | 26.72 | 29.49 | 53.29 | 75.20 |
| 0.1 | 34.42 | 24.86 | 48.98 | 140.45 |
| 0.2 | 37.34 | 41.72 | 47.67 | 152.33 |
| 0.3 | 45.94 | 40.62 | 44.49 | 183.93 |
| 0.4 | 48.72 | 40.50 | 43.63 | 214.99 |
| Temperature (°C) | (Pa) | (s) | (nm) | (Pa) |
|---|---|---|---|---|
| 20 | 26.73 | 29.49 | 53.29 | 75.20 |
| 25 | 25.03 | 8.73 | 54.78 | 69.50 |
| 30 | 26.30 | 11.12 | 54.19 | 57.96 |
| 35 | 27.63 | 8.02 | 53.59 | 46.21 |
| 40 | 29.27 | 4.74 | 52.85 | 26.73 |
| 45 | 30.86 | 1.91 | 52.20 | 12.44 |
| Temperature (°C) | (Pa) | (s) | (nm) | (Pa) |
|---|---|---|---|---|
| 20 | 48.72 | 40.50 | 43.63 | 214.99 |
| 25 | 53.01 | 25.41 | 42.66 | 204.31 |
| 30 | 54,70 | 13.48 | 42.45 | 115.53 |
| 35 | 56,21 | 5.78 | 42.30 | 70.61 |
| 40 | 58.04 | 1.33 | 42.08 | 27.63 |
| 45 | 47.20 | 0.37 | 45.31 | 11.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silin, M.; Magadova, L.; Poteshkina, K.; Krisanova, P.; Filatov, A.; Kryukov, D. Experimental Investigation of Hydraulic Fracturing Fluid Based on Pseudo Gemini Surfactant with Polysaccharide Addition. Gels 2024, 10, 30. https://doi.org/10.3390/gels10010030
Silin M, Magadova L, Poteshkina K, Krisanova P, Filatov A, Kryukov D. Experimental Investigation of Hydraulic Fracturing Fluid Based on Pseudo Gemini Surfactant with Polysaccharide Addition. Gels. 2024; 10(1):30. https://doi.org/10.3390/gels10010030
Chicago/Turabian StyleSilin, Mihail, Lyubov Magadova, Kira Poteshkina, Polina Krisanova, Andrey Filatov, and Denis Kryukov. 2024. "Experimental Investigation of Hydraulic Fracturing Fluid Based on Pseudo Gemini Surfactant with Polysaccharide Addition" Gels 10, no. 1: 30. https://doi.org/10.3390/gels10010030