Exploring the Potential of Atlantic Mesopelagic Species Processed on Board Commercial Fishing Vessels as a Source of Dietary Lipids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sampling Procedure
2.3. Proximate Composition
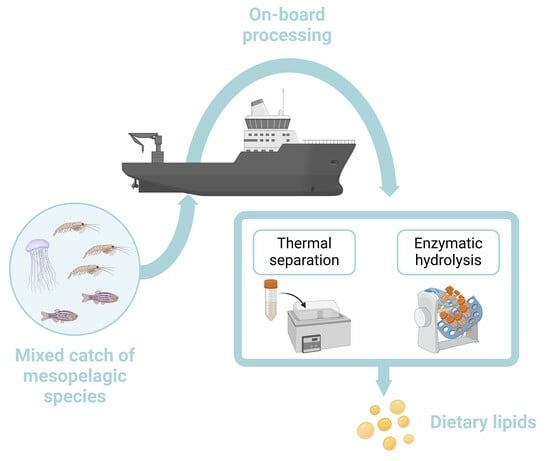
2.4. Processing Technologies on Board Commercial Vessels
2.5. Thermal Separation
2.6. Enzymatic Hydrolysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Mixed Catches of Mesopelagic Species
3.2. Lipid Classes and Fatty Acid Composition
3.3. Thermal and Enzymatic Processing of Mesopelagic Species
3.4. Free Fatty Acids as Quality Indicators
3.5. Mass Distribution of Lipids and LC n-3 PUFAs
3.6. Dietary Lipids from the Mesopelagic Biomass
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisson, K.; McMonagle, H.; Iglesias, I.; Halfter, S.; Gallo, N. Five Reasons to Take the Precautionary Approach to Deep Sea Exploitation. Commun. Earth Environ. 2023, 4, 152. [Google Scholar] [CrossRef]
- Grimaldo, E.; Grimsmo, L.; Alvarez, P.; Herrmann, B.; Møen Tveit, G.; Tiller, R.; Slizyte, R.; Aldanondo, N.; Guldberg, T.; Toldnes, B.; et al. Investigating the Potential for a Commercial Fishery in the Northeast Atlantic Utilizing Mesopelagic Species. ICES J. Mar. Sci. 2020, 77, 2541–2556. [Google Scholar] [CrossRef]
- Alvheim, A.R.; Kjellevold, M.; Strand, E.; Sanden, M.; Wiech, M. Mesopelagic Species and Their Potential Contribution to Food and Feed Security—A Case Study from Norway. Foods 2020, 9, 344. [Google Scholar] [CrossRef]
- Salas-Maldonado, A.; Ayala-Galdós, M.E.; Albrecht-Ruiz, M. Contenido De Epa Y Dha En Aceite Crudo De Pescado Producido En El Perú Durante El Periodo 1996–2000 Content of Epa and Dha in Raw Fish Oil Produced in Peru During 1996–2000 Contido De Epa Y Dha En Aceite Cru De Pescado Producido En El Perú Durante El Período 1996–2000. Cienc. Tecnol. Aliment. 2002, 3, 283–287. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPAand DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef]
- Fjeld, K.; Tiller, R.; Grimaldo, E.; Grimsmo, L.; Standal, I.-B. Mesopelagics–New Gold Rush or Castle in the Sky? Mar. Policy 2023, 147, 105359. [Google Scholar] [CrossRef]
- Pauly, D.; Piroddi, C.; Hood, L.; Bailly, N.; Chu, E.; Lam, V.; Pakhomov, E.A.; Pshenichnov, L.K.; Radchenko, V.I.; Palomares, M.L.D. The Biology of Mesopelagic Fishes and Their Catches (1950–2018) by Commercial and Experimental Fisheries. J. Mar. Sci. Eng. 2021, 9, 1057. [Google Scholar] [CrossRef]
- Prellezo, R. Exploring the Economic Viability of a Mesopelagic Fishery in the Bay of Biscay. ICES J. Mar. Sci. 2019, 76, 771–779. [Google Scholar] [CrossRef]
- Bergvik, M.; Overrein, I.; Bantle, M.; Evjemo, J.O.; Rustad, T. Properties of Calanus Finmarchicus Biomass during Frozen Storage after Heat Inactivation of Autolytic Enzymes. Food Chem. 2012, 132, 209–215. [Google Scholar] [CrossRef]
- Albrektsen, S.; Kortet, R.; Skov, P.V.; Ytteborg, E.; Gitlesen, S.; Kleinegris, D.; Mydland, L.-T.; Hansen, J.Ø.; Lock, E.-J.; Mørkøre, T.; et al. Future Feed Resources in Sustainable Salmonid Production: A Review. Rev. Aquac. 2022, 14, 1790–1812. [Google Scholar] [CrossRef]
- Sajib, M.; Trigo, J.P.; Abdollahi, M.; Undeland, I. Pilot-Scale Ensilaging of Herring Filleting Co-Products and Subsequent Separation of Fish Oil and Protein Hydrolysates. Food Bioprocess Technol. 2022, 15, 2267–2281. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Schulte, H.; Pleissner, D.; Schönfelder, S.; Kvangarsnes, K.; Dauksas, E.; Rustad, T.; Cropotova, J.; Heinz, V.; Smetana, S. Transformation of Seafood Side-Streams and Residuals into Valuable Products. Foods 2023, 12, 422. [Google Scholar] [CrossRef]
- Olsen, R.E.; Strand, E.; Melle, W.; Nørstebø, J.T.; Lall, S.P.; Ringø, E.; Tocher, D.R.; Sprague, M. Can Mesopelagic Mixed Layers Be Used as Feed Sources for Salmon Aquaculture? Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 180, 104722. [Google Scholar] [CrossRef]
- Naik, A.S.; Whitaker, R.D.; Albrektsen, S.; Solstad, R.G.; Thoresen, L.; Hayes, M. Mesopelagic Fish Protein Hydrolysates and Extracts: A Source of Novel Anti-Hypertensive and Anti-Diabetic Peptides. Front. Mar. Sci. 2021, 8, 719608. [Google Scholar] [CrossRef]
- Wiech, M.; Silva, M.; Meier, S.; Tibon, J.; Berntssen, M.H.G.; Duinker, A.; Sanden, M. Undesirables in Mesopelagic Species and Implications for Food and Feed Safety—Insights from Norwegian Fjords. Foods 2020, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Gatto, A.; Sadik-Zada, E.R.; Özbek, S.; Kieu, H.; Nguyen Huynh, N.T. Deep-Sea Fisheries as Resilient Bioeconomic Systems for Food and Nutrition Security and Sustainable Development. Resour. Conserv. Recycl. 2023, 197, 106907. [Google Scholar] [CrossRef]
- Sajib, M.; Langeland, M.; Undeland, I. Effect of Antioxidants on Lipid Oxidation in Herring (Clupea Harengus) Co-Product Silage during Its Production, Heat-Treatment and Storage. Sci. Rep. 2022, 12, 3362. [Google Scholar] [CrossRef]
- Remme, J.F.; Korsnes, S.; Steen, S.; Durand, R.; Kvangarsnes, K.; Stangeland, J. The Effects of Enzymes, Species, and Storage of Raw Material on Physicochemical Properties of Protein Hydrolysates from Whitefish Heads. Mar. Drugs 2023, 21, 587. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Grokhovsky, V.A.; Kuranova, L.K.; Volchenko, V.I. Nutrient Analysis of Underutilized Fish Species for the Production of Protein Food. Foods Raw Mater. 2017, 5, 15–23. [Google Scholar] [CrossRef]
- Albessard, E.; Mayzaud, P.; Cuzin-Roudy, J. Variation of Lipid Classes among Organs of the Northern Krill Meganyctiphanes Norvegica, with Respect to Reproduction. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 129, 373–390. [Google Scholar] [CrossRef]
- Shepherd, C.J.; Jackson, A.J. Global Fishmeal and Fish-Oil Supply: Inputs, Outputs and Marketsa. J. Fish Biol. 2013, 83, 1046–1066. [Google Scholar] [CrossRef]
- Cook, C.M.; Larsen, T.S.; Derrig, L.D.; Kelly, K.M.; Tande, K.S. Wax Ester Rich Oil from the Marine Crustacean, Calanus Finmarchicus, Is a Bioavailable Source of EPA and DHA for Human Consumption. Lipids 2016, 51, 1137–1144. [Google Scholar] [CrossRef]
- Szlinder-Richert, J.; Usydus, Z.; Wyszyński, M.; Adamczyk, M. Variation in Fat Content and Fatty-Acid Composition of the Baltic Herring Clupea Harengus Membras. J. Fish Biol. 2010, 77, 585–599. [Google Scholar] [CrossRef]
- El, O.S.; Darej, C.; Moujahed, N. Fatty Acids and Amino Acids Contents in Scomber scombrus Fillets from the South East of Tunisia. Afr. J. Biotechnol. 2016, 15, 1246–1252. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- Liu, Y.; Ramakrishnan, V.V.; Dave, D. Lipid Class and Fatty Acid Composition of Oil Extracted from Atlantic Salmon By-Products under Different Optimization Parameters of Enzymatic Hydrolysis. Biocatal. Agric. Biotechnol. 2020, 30, 101866. [Google Scholar] [CrossRef]
- Chiodza, K.; Goosen, N.J. Emulsion Formation during Enzymatic Protein Hydrolysis and Its Effect on Protein Recovery and Molecular Weight Distribution of Protein Hydrolysates from Sardine (Sardina pilchardus) by-Products. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on B. Scientific Opinion on Fish Oil for Human Consumption. Food Hygiene, Including Rancidity. EFSA J. 2010, 8, 1874. [Google Scholar] [CrossRef]
- Global Organization for EPA and DHA Omega-3s. GOED Voluntary Monograph, Version 6; GOED: Salt Lake City, UT, USA, 2018. [Google Scholar]
- Rudy, M.D.; Kainz, M.J.; Graeve, M.; Colombo, S.M.; Arts, M.T. Handling and Storage Procedures Have Variable Effects on Fatty Acid Content in Fishes with Different Lipid Quantities. PLoS ONE 2016, 11, e0160497. [Google Scholar] [CrossRef]
- Reguleringsmøte November 2023. Available online: https://www.fiskeridir.no/Yrkesfiske/Dokumenter/Reguleringsmoetet2/november-2023 (accessed on 25 February 2024).
- Falch, E.; Rustad, T.; Aursand, M. By-Products from Gadiform Species as Raw Material for Production of Marine Lipids as Ingredients in Food or Feed. Process Biochem. 2006, 41, 666–674. [Google Scholar] [CrossRef]
- Salem, N.; Eggersdorfer, M. Is the World Supply of Omega-3 Fatty Acids Adequate for Optimal Human Nutrition? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 147–154. [Google Scholar] [CrossRef]
- Hamilton, H.A.; Newton, R.; Auchterlonie, N.A.; Müller, D.B. Systems Approach to Quantify the Global Omega-3 Fatty Acid Cycle. Nat. Food 2020, 1, 59–62. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Fats, Including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health Text with EEA Relevance. 2012, Volume 136. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32012R0432 (accessed on 1 February 2024).
- Irigoien, X.; Klevjer, T.A.; Røstad, A.; Martinez, U.; Boyra, G.; Acuña, J.L.; Bode, A.; Echevarria, F.; Gonzalez-Gordillo, J.I.; Hernandez-Leon, S.; et al. Large Mesopelagic Fishes Biomass and Trophic Efficiency in the Open Ocean. Nat. Commun. 2014, 5, 3271. [Google Scholar] [CrossRef]
- Dietary Reference Values for Fats|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/1461 (accessed on 11 February 2024).




| Cruise No. | Station | Date (DD MM YYYY) | Time (UTC) | Trawling Time (min) | Position | Fishing Depth (m) |
|---|---|---|---|---|---|---|
| Cruise 1 | 1 | 28 June 2016 | 21:00 | 30 | 60°30′ N 04°37′ W | 220 |
| 2 | 2 July 2016 | 18:16 | 60 | 59°46′ N 31°26′ W | 480 | |
| 3 | 3 July 2016 | 18:09 | 50 | 56°30′ N 31°27′ W | 400 | |
| 4 | 4 July 2016 | 20:55 | 40 | 53°46′ N 32°00′ W | 400 | |
| 5 | 5 July 2016 | 13:00 | 30 | 52°16′ N 25°57′ W | 400 | |
| 6 | 5 July 2016 | 20:15 | 15 | 51°37′ N 26°33′ W | 160 | |
| 7 | 7 July 2016 | 08:30 | 30 | 46°33′ N 26°01′ W | 150 | |
| 8 | 7 July 2016 | 13:15 | 30 | 46°09′ N 26°16′ W | 500 | |
| 9 | 13 July 2016 | 17:55 | 20 | 37°34′ N 19°15′ W | 470 | |
| 10 | 14 July 2016 | 17:00 | 15 | 38°46′ N 15°26′ W | 250 | |
| Cruise 2 | 1 | 21 April 2017 | 17:52 | 20 | 53°51′ N 17°19 W | 100 |
| 3 | 30 April 2017 | 18:00 | 60 | 48°13′ N 26°19′ W | 320 | |
| 4 | 5 May 2017 | 11:53 | 45 | 43°27′ N 26°41′ W | 50 | |
| 5 | 9 May 2017 | 17:52 | 45 | 55°34′ N 20°09′ W | 199 | |
| Cruise 3 | 1 | 16 July 2017 | 10:10 | 130 | 47°10′ N 26°17′ W | 260 |
| 2 | 16 July 2017 | 18:47 | 45 | 46°15′ N 26°33′ W | 212 | |
| 3 | 17 July 2017 | 14:30 | 50 | 44°20′ N 29°01′ W | 338 | |
| 4 | 18 July 2017 | 18:53 | 50 | 48°00′ N 26°43′ W | 212 | |
| 5 | 19 July 2017 | 20:09 | 60 | 45°47′ N 21°36′ W | 85 | |
| 6 | 21 July 2017 | 16:25 | 50 | 39°24′ N 16°54′ W | 212 | |
| 7 | 23 July 2017 | 15:27 | 60 | 42°24′ N 16°02′ W | 170 | |
| 8 | 29 July 2017 | 13:20 | 30 | 46°52′ N 10°14′ W | 100 | |
| Cruise 4 | 1 | 7 November 2019 | 11:45 | 50 | 59°25′ N 17°59′ W | 180 |
| 2 | 11 November 2019 | 10:45 | 60 | 59°25′ N 17°59′ W | 245 | |
| 3 | 13 November 2019 | 09:30 | 122 | 59°25′ N 17°59′ W | 269 | |
| 4 | 13 November 2019 | 13:40 | 60 | 59°25′ N 17°59′ W | 266 | |
| 5 | 14 November 2019 | 10:12 | 73 | 59°25′ N 17°59′ W | 252 | |
| 6 | 18 November 2019 | 11:52 | 61 | 59°25′ N 17°59′ W | 241 | |
| 7 | 19 November 2019 | 10:32 | 67 | 59°25′ N 17°59′ W | 244 |
| Cruise | Lipid | Proteins (N × 6.25) | Ash | Moisture | B. glaciale | M. muelleri | Krill | Others |
|---|---|---|---|---|---|---|---|---|
| Cruise 1 | 9.9 ± 3.9 | 14.6 ± 1.2 | 2.9 ± 0.2 | 72.4 ± 3.0 | 41 | 42.6 | 10 | 5.9 |
| Cruise 2 | 2.3 ± 0.7 | 14.5 ± 2.8 | 3.2 ± 0.4 | 79.8 ± 2.7 | 31 | 54.2 | 34 | 9.2 |
| Cruise 3 | 4.9 ± 2.6 | 15.3 ± 1.5 | 3.3 ± 0.3 | 76.4 ± 2.8 | 7.8 | 64.5 | 25 | 2.6 |
| Cruise 4 | 9.8 ± 2.4 | 11.8 ± 1.3 | 3.0 ± 0.1 | 75.7 ± 2.3 | 69.3 | 9 | 21.8 |
| Cruise 1 | Cruise 2 | Cruise 3 | Cruise 4 | p < 0.05 | ||
|---|---|---|---|---|---|---|
| Lipid content (%) | 9.9 (4.3–15.8) | 2.3 (1.4–3.0) | 4.9 (2.2–9.5) | 9.8 (5.6–12.4) | ||
| Lipid classes (% total lipids) | Wax esters | 39.5 (0–85.4) | 8.3 (0.6–29.4) | 10.0 (0–40.2) | - | - |
| Triacylglycerols | 45.6 (3.3–89.9) | 23.9 (5.0–54.4) | 44.3 (11.3–77.5) | - | - | |
| Free fatty acids | 1.8 (0.5–4.4) | 27.9 (21.1–25.9) | 13.3 (3.1–25.3) | - | C2 > C3 > C1 | |
| Cholesterol | 1.6 (0.5–4.3) | 11.1 (6.3–16.2) | 6.8 (2.6–11.4) | - | C2 > C3 > C1 | |
| Phospholipids | 16.5 (8.9–38.3) | 28.8 (17.1–41.1) | 24.5 (15.6–29.5) | - | C2 > C1 |
| Fatty Acid | Cruise 1 | Cruise 2 | Cruise 3 | Cruise 4 | p < 0.05 |
|---|---|---|---|---|---|
| C14:0 | 5.0 (2.9–7.2) | 5.2 (3.3–6.7) | 4.7 (2.4–8.9) | 6.9 (4.8–7.8) | - |
| C14:1 | 0.2 (0–0.3) | 0.2 (0.1–0.3) | 0.3 (0.3–0.4) | 0.4 (0.3–0.4) | C1 < C3; C1, C2 < C4 |
| C15:0 | 0.5 (0.2–0.8) | 0.5 (0.4–0.6) | 0.8 (0.5–1.1) | 0.8 (0.8–0.8) | C1 < C3, C4 |
| C16:0 | 15.5 (6.8–29.4) | 19.6 (16.6–22.4) | 25.3 (14.9–37.2) | 21.4 (18.9–26.8) | - |
| C16:1 n7 + n9 | 6.5 (3.1–10.5) | 4.8 (3.5–6.2) | 3.8 (2.3–6.4) | 4.4 (3.9–4.7) | C1 > C3 |
| C17:0 | 0.5 (0.2–0.7) | 0.7 (0.5–1.0) | 0.8 (0.6–0.9) | 0.6 (0.4–0.8) | C1 < C3 |
| C17:1 | 0.5 (0.4–0.7) | 0.7 (0.5–1.0) | 0.7 (0.6–1.0) | 0.5 (0.4–0.8) | C1 < C3 |
| C18:0 | 2.5 (1.5–4.9) | 3.1 (1.7–4.4) | 3.9 (2.8–5.6) | 2.3 (2.0–2.8) | C1, C4 < C3 |
| C18:1 n11 + n9 | 18.5 (8.3–25.8) | 13.1 (7.9–24.9) | 16.8 (12.7–22.1) | 11.5 (9.8–16.4) | - |
| C18:1 n7 | 1.9 (1.3–2.5) | 2.7 (2.3–3.0) | 1.4 (0.5–2.5) | 1.7 (1.3–2.2) | C1, C3, C4 < C2 |
| C18:2 n6 | 1.5 (1.2–1.9) | 1.6 (1.2–2.0) | 1.6 (0.9–1.9) | 1.3 (1.2–1.5) | - |
| C18:3 n6 | 0.2 (0.1–0.2) | 0.1 (0.1–0.2) | 0.2 (0.1–0.3) | 0.2 (0.2–0.3) | - |
| C18:3 n3 | 0.8 (0–1.4) | 0.8 (0.5–1.2) | 0.8 (0.6–1.1) | 1.2 (0.9–1.5) | - |
| c18:4 n3 | 2.3 (0.5–3.8) | 1.7 (0.7–3.5) | 2.2 (1.5–2.9) | 3.7 (2.8–4.6) | C1, C2, C3 < C4 |
| C20:0 | 0.2 (0.1–0.3) | 0.1 (0.1–0.1) | 0.2 (0.1–0.3) | 0.2 (0.2–0.2) | CC1, C3, C4 > C2 |
| C20:1 n11+ n9 +n7 | 7.7 (1.8–11.8) | 3.8 (1.7–9.3) | 1.2 (0.4–2.0) | 6.1 (1.6–8.1) | C1, C4 > C3 |
| C20:2 n6 | 0.3 (0.2–0.4) | 0.2 (0.2–0.3) | 0.3 (0.4–0.4) | 0.2 (0.2–0.3) | - |
| C20:3 n6 | 0.1 (0–0.2) | 0.1 (0.1–0.2) | 0.1 (0–0.1) | 0.1 (0.1–0.1) | - |
| C20:4 n6 | 0.2 (0.1–0.3) | 0.7 (0.5–0.8) | 0.4 (0.2–0.7) | 0.1 (0–0.1) | C1, C4 < C2 < C3 |
| C20:3 n3 | 0.1 (0–0.1) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.4) | C1 < C3, C4 |
| C20:4 n3 | 0.9 (0.6–1.3) | 0.5 (0.1–0.7) | 0.9 (0.4–1.0) | 0.9 (0.8–1.1) | - |
| C20:5 n3 EPA | 7.1 (5.1–10.1) | 9.8 (8.3–11) | 7.5 (3.4–10) | 7.4 (6.3–8.5) | - |
| C22:0 | 0.1 (0–0.2) | 0.1 (0.1–0.1) | 0.2 (0.1–0.2) | 0.1 (0–0.2) | - |
| c22:1 n11 | 9.2 (0.8–18.6) | 4.8 (0.8–13.6) | 0.3 (0.1–0.7) | 8.8 (0.5–12.6) | C1, C4 > C3 |
| C22:1 n9 | 0.6 (0.2–1.0) | 0.3 (0.1–0.6) | 0.2 (0.1–0.3) | 0.4 (0.2–0.5) | C1 > C3 |
| C22:2 | 0.1 (0–0.4) | 0.3 (0.3–0.5) | 0.3 (0.2–0.4) | 0.4 (0.2–0.4) | C1 < C2, C3, C4 |
| C22:3 | 0.1 (0–0.1) | 0.1 (0–0.1) | 0.1 (0–0.1) | 0.0 (0–0.1) | - |
| C22:4 | 0.4 (0.2–0.7) | 0.4 (0.2–0.5) | 0.7 (0.4–1.0) | 0.3 (0.2–0.3) | C1, C2, C4 < C3 |
| c22:5 n3 | 0.8 (0.7–1.0) | 0.9 (0.4–1.4) | 0.6 (0.3–0.7) | 0.8 (0.7–0.9) | - |
| C24:0 | 0.0 - | 0.0 - | 0.0 - | 0.0 - | |
| C22:6 n3 DHA | 14.7 (8–25.7) | 22.0 (13.6–31.0) | 22.4 (11.3–27.1) | 16.1 (13.3–20) | C1 < C3 |
| C24:1 n9 | 1.1 (0.8–1.6) | 0.9 (0.5–1.4) | 1.2 (0.6–1.6) | 1.0 (0.8–1.2) | - |
| SFAs | 24.2 (13.9–38.4) | 29.3 (25.0–33.2) | 35.9 (21.7–53.7) | 32.3 (30.1–36.5) | C1 < C3 |
| MUFAs | 46.3 (24.8–61.9) | 31.2 (21.0–39.7) | 26.1 (19.5–36.6) | 34.8 (26.9–39.7) | C1 > C2, C3 |
| PUFAs | 29.5 (22.0–43.5) | 39.5 (33.2–47.1) | 38.0 (19.9–44.6) | 32.9 (28.4–36.5) | C1 < C2, C3 |
| n-3 | 26.6 (16.5–37.9) | 36.0 (30.1–43.9) | 34.6 (18.0–40.7) | 30.3 (26.2–34.1) | C1 < C2, C3 |
| Cruise | Lipid Content | Fractions | Thermal Separation | Endogenous Enzymes | Papain-Bromelain | Protamex | Corolase | p < 0.05 |
|---|---|---|---|---|---|---|---|---|
| Cruise 1 | 9.9 (4.3–15.8) | Oil | 9.6 (5.3–12.7) | 7.4 (2.9–9.4) | 7.2 (2.3–11.5) | 5.9 (2.4–7.6) | 6.3 (2.6–7.8) | |
| Emulsion | - | 7.9 (0–16.7) | 13.4 (3.2–19.5) | 17.9 (8.6–24.1) | 17.5 (6.5–23.1) | |||
| Stickwater/Protein hydrolysate | 34.9 (31.1–44.7) | 17.9 (13.1–26.3) | 28.2 (24.9–36.1) | 27.6 (24.5–32.8) | 27.5 (24.8–31.4) | T > E < PB, P, C | ||
| Sludge/Sediments | 55.5 (49.4–58.5) | 66.8 (60.0–70.7) | 51.2 (47.6–57.5) | 48.6 (40.8–54.7) | 48.7 (43.6–56.1) | T > E < PB, P, C | ||
| Cruise 2 | 2.3 (1.4–3.0) | Oil | 1.3 (0–6.3) | - | - | - | - | |
| Emulsion | - | - | - | - | ||||
| Stickwater/Protein hydrolysate | 42.7 (31.8–67.8) | - | - | - | - | |||
| Sludge/Sediments | 56 (32.2–68.1) | - | - | - | - | |||
| Cruise 3 | 4.9 (2.2–9.5) | Oil | 1.3 (0–6.3) | - | - | - | - | |
| Emulsion | - | - | - | - | - | |||
| Stickwater/Protein hydrolysate | 39.6 (29.2–45.1) | - | - | - | - | |||
| Sludge/Sediments | 59.1 (54.4–67.9) | - | - | - | - | |||
| Cruise 4 | 9.8 (5.6–12.4) | Oil | 6.8 (2.9–8.4) | 6.6 (1.0–10.7) | 9 (1.6–12.2) | 7.7 (1.1–12.0) | - | |
| Emulsion | - | - | - | - | - | |||
| Stickwater/Protein hydrolysate | 42 (30.1–55.8) | 36.4 (29–46.3) | 39.2 (36.9–42.1) | 38.1 (32.2–42.3) | - | |||
| Sludge/Sediments | 51.2 (35.7–61.7) | 57 (45.4–62.0) | 51.8 (45.9–58.9) | 54.2 (45.9–57.8) | - |
| Cruise | Haul | Total Catch (kg) | Lipids | n-3 in Lipids | n-3 in Raw Material | ||
|---|---|---|---|---|---|---|---|
| % | kg | % | kg | % | |||
| Cruise 1 | 1 | 4000 | 15.8 ± 0.0 | 632.4 | 19.4 ± 0.1 | 122.4 | 3.1 |
| 2 | 1000 | 11.0 ± 0.6 | 110.1 | 18.9 ± 0.2 | 20.9 | 2.1 | |
| 3 | 1000 | 13.7 ± 0.4 | 137.2 | 28.3 ± 0.2 | 38.8 | 3.9 | |
| 4 | 1000 | 12.0 ± 0.3 | 120.4 | 27.5 ± 0.1 | 33.0 | 3.3 | |
| 5 | 1000 | 12.2 ± 0.2 | 122.3 | 26.7 ± 0.1 | 32.6 | 3.3 | |
| 6 | 2000 | 6.2 ± 0.2 | 123.8 | 24.3 ± 0.1 | 30.1 | 1.5 | |
| 7 | 1000 | 4.3 ± 0.2 | 43.4 | 40.3 ± 0.1 | 17.5 | 1.7 | |
| 8 | 500 | 7.0 ± 0.0 | 35.15 | 26.3 ± 0.3 | 9.2 | 1.8 | |
| 9 | 100 | 5.4 ± 0.0 | 5.4 | 25.3 ± 0.2 | 1.4 | 1.4 | |
| 10 | 3000 | 11.1 ± 0.1 | 333.3 | 29.7 ± 0.0 | 98.9 | 3.3 | |
| Average | 1460 | 9.9 ± 0.2 | 166.3 | 26.6 ± 0.2 | 40.5 | 2.5 | |
| Sum | 14,600 | 1663 | 405 | ||||
| Cruise 2 | 1 | 200 | 1.9 ± 0.1 | 3.8 | 44.0 ± 0.4 | 1.7 | 0.8 |
| 3 | 350 | 1.4 ± 0.0 | 5.0 | 31.7 ± 0.1 | 1.6 | 0.5 | |
| 4 | 600 | 2.6 ± 0.1 | 15.8 | 38.1 ± 01 | 6.0 | 1.0 | |
| 5 | 500 | 3.0 ± 0.1 | 15.2 | 30.1 ± 0.1 | 4.6 | 0.9 | |
| Average | 515 | 2.3 ± 0.1 | 12.5 | 35.9 ± 0.2 | 3.5 | 0.8 | |
| Sum | 2060 | 50 | 14 | ||||
| Cruise 3 | 1 | 300 | 4.3 ± 0.1 | 13.0 | 36.9 ± 0.0 | 4.8 | 1.6 |
| 2 | 275 | 2.2 ± 0.0 | 6.1 | 39.5 ± 0.1 | 2.4 | 0.9 | |
| 3 | 100 | 3.2 ± 0.0 | 3.2 | 38.0 ± 0.5 | 1.2 | 1.2 | |
| 4 | 250 | 5.8 ± 0.0 | 14.6 | 37.8 ± 0.0 | 5.5 | 2.2 | |
| 5 | 300 | 3.7 ± 0.2 | 11.0 | 37.8 ± 0.3 | 4.2 | 1.4 | |
| 6 | 100 | 9.5 ± 0.1 | 9.5 | 30.3 ± 0.3 | 2.9 | 2.9 | |
| 7 | 300 | 2.5 ± 0.0 | 7.4 | 40.7 ± 0.0 | 3.0 | 1.0 | |
| 8 | 100 | 7.8 ± 0.2 | 7.8 | 34.7 ± 0.0 | 2.7 | 2.7 | |
| Average | 215.6 | 4.9 ± 0.1 | 9.1 | 37.0 ± 0.2 | 3.3 | 1.7 | |
| Sum | 1725 | 73 | 26 | ||||
| Cruise 4 | 1 | 30.3 | 10.8 ± 0.2 | 3.3 | 34.1 ± 0.6 | 1.1 | 3.7 |
| 2 | 90 | 12.4 ± 0.3 | 11.2 | 28.1 ± 0.1 | 3.1 | 3.5 | |
| 3 | 500 | 9.2 ± 0.1 | 45.9 | 33.0 ± 0.1 | 15.2 | 3.0 | |
| 4 | 500 | 8.3 ± 0.1 | 41.3 | 32.5 ± 0.1 | 13.4 | 2.7 | |
| 5 | 42 | 12.2 ± 0.1 | 5.1 | 26.2 ± 0.1 | 1.3 | 3.2 | |
| 6 | 110.1 | 9.8 ± 0.1 | 10.8 | 29.8 ± 0.1 | 3.2 | 2.9 | |
| 7 | 209.9 | 5.6 ± 0.1 | 11.8 | 28.7 ± 0.2 | 3.4 | 1.6 | |
| Average | 211.8 | 9.8 ± 0.1 | 18.5 | 30.3 ± 0.2 | 5.8 | 2.9 | |
| Sum | 1483 | 130 | 41 | ||||
| Total sum | 19,868 | 1915 | 486 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madina, M.A.; Grimaldo, E.; Grimsmo, L.; Toldnes, B.; Slizyte, R.; Carvajal, A.K.; Schei, M.; Selnes, M.; Falch, E. Exploring the Potential of Atlantic Mesopelagic Species Processed on Board Commercial Fishing Vessels as a Source of Dietary Lipids. Foods 2024, 13, 1094. https://doi.org/10.3390/foods13071094
Madina MA, Grimaldo E, Grimsmo L, Toldnes B, Slizyte R, Carvajal AK, Schei M, Selnes M, Falch E. Exploring the Potential of Atlantic Mesopelagic Species Processed on Board Commercial Fishing Vessels as a Source of Dietary Lipids. Foods. 2024; 13(7):1094. https://doi.org/10.3390/foods13071094
Chicago/Turabian StyleMadina, Maria A., Eduardo Grimaldo, Leif Grimsmo, Bendik Toldnes, Rasa Slizyte, Ana Karina Carvajal, Marte Schei, Merethe Selnes, and Eva Falch. 2024. "Exploring the Potential of Atlantic Mesopelagic Species Processed on Board Commercial Fishing Vessels as a Source of Dietary Lipids" Foods 13, no. 7: 1094. https://doi.org/10.3390/foods13071094