The Combined Antioxidant Effects of N-Acetylcysteine, Vitamin D3, and Glutathione from the Intestinal–Neuronal In Vitro Model
Abstract
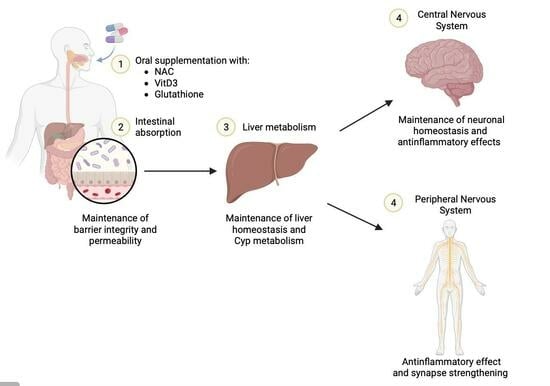
:1. Introduction
2. Materials and Methods
2.1. Reagent Preparations
2.2. Cell Cultures
2.3. Experimental Protocol
2.4. In Vitro Model of the Intestinal Barrier
2.5. In Vitro Hepatic Environment
2.6. BBB-CNS Axis
2.7. PNS In Vitro Model
2.8. MTT Viability
2.9. ROS Production
2.10. TNFα Assay
2.11. NFkB Analysis
2.12. Interleukin 1β Assay
2.13. CYP1A2 Assay
2.14. CYP3A4 Assay
2.15. Src Activity
2.16. ERK/MAPK Activity
2.17. Amyloid Beta A4 ELISA Kit
2.18. pTAU Assay
2.19. p75 Activity by NGFR ELISA Assay Kit
2.20. Myelin Protein Zero Detection
2.21. NRG1 Assay
2.22. Oestrogen Receptor Beta Activity
2.23. Statistical Analysis
3. Results
3.1. Cell Viability of NAC, VitD3, and Glut on CaCo-2 Cells
3.2. NAC, VitD3, and Glut Permeability and Absorption Analysis through Intestinal Barrier
3.3. NAC, VitD3, and Glut Role in the Hepatic Cell Line
3.4. Beneficial Effects of NAC, VitD3, and Glut on Astrocytes Crossing the BBB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | one-way analysis of variance |
| ATCC | American Type Culture Collection |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| DMEM-Adv | Dulbecco’s Modified Eagle’s Medium Advance |
| EGM-2 | Endothelial Growth Medium-2 |
| EMA | European Medicines Agency |
| ERK | extracellular signal-regulated kinase |
| FBS | Foetal Bovine Serum |
| FDA | Food and Drug Administration |
| GA-1000 | gentamicin sulphate-amphotericin |
| hEGF | human epidermal growth factor |
| hFGF-B | human basic fibroblast growth factor |
| HS | horse serum |
| IL | Interleukin |
| MEM | minimum essential medium |
| MAPK | mitogen-activated protein kinase |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAC | N-acetylcysteine |
| Papp | apparent permeability coefficient |
| PNS | peripheral nervous system |
| R3-IGF-1 | insulin-like growth factor-I |
| ROS | reactive oxygen species |
| RPMI | Roswell Park Memorial Institute medium |
| TEER | transepithelial electrical resistance |
| TNFα | tumour necrosis factor alpha |
| VEGF | vascular endothelial growth factor |
| VitD3 | vitamin D3 |
Appendix A

References
- Stefanatos, R.; Sanz, A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Munshi, S.; Banerjee, K.; Thakurta, I.G.; Sinha, M.; Bagh, M.B. Mitochondrial Dysfunction during Brain Aging: Role of Oxidative Stress and Modulation by Antioxidant Supplementation. Aging Dis. 2011, 2, 242–256. [Google Scholar] [PubMed]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; Al Salamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Hernández, A.F.; Gil, F. Nutraceuticals and adverse outcome pathways. In Nutraceuticals, 2nd ed.; Gupta, R., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1159–1174. [Google Scholar]
- Khalaf, A.T.; Wei, Y.; Alneamah, S.J.A.; Al-Shawi, S.G.; Kadir, S.Y.A.; Zainol, J.; Liu, X. What Is New in the Preventive and Therapeutic Role of Dairy Products as Nutraceuticals and Functional Foods? Biomed. Res. Int. 2021, 2021, 8823222. [Google Scholar] [CrossRef]
- Montagna, M.T.; Diella, G.; Triggiano, F.; Caponio, G.R.; De Giglio, O.; Caggiano, G.; Di Ciaula, A.; Portincasa, P. Chocolate, “Food of the Gods”: History, Science, and Human Health. Int. J. Environ. Res. Public. Health 2019, 16, 4960. [Google Scholar] [CrossRef]
- Crichton, G.E.; Bryan, J.; Murphy, K.J. Dietary Antioxidants, Cognitive Function and Dementia—A Systematic Review. Plant Foods Hum. Nutr. 2013, 68, 279–292. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert. Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, M.; Ito, Y. N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum 2007, 6, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Teder, K.; Maddison, L.; Soeorg, H.; Meos, A.; Karjagin, J. The Pharmacokinetic Profile and Bioavailability of Enteral N-Acetylcysteine in Intensive Care Unit. Medicina 2021, 57, 1218. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Birdsall, T.C.; Grutsch, J.F. The use of dietary supplements in a community hospital comprehensive cancer center: Implications for conventional cancer care. Support. Care Cancer 2005, 13, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.M.; Meléndez, C.M.; Uribe, D.; Pedroza-Díaz, J. Synergistic effects of natural compounds and conventional chemotherapeutic agents: Recent insights for the development of cancer treatment strategies. Heliyon 2022, 8, e09519. [Google Scholar] [CrossRef]
- Carota, G.; Distefano, A.; Spampinato, M.; Giallongo, C.; Broggi, G.; Longhitano, L.; Palumbo, G.A.; Parenti, R.; Caltabiano, R.; Giallongo, S.; et al. Neuroprotective Role of α-Lipoic Acid in Iron-Overload-Mediated Toxicity and Inflammation in In Vitro and In Vivo Models. Antioxidants 2022, 11, 1596. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, C.; Peng, M.; Wang, L.; Zhao, D.; Wu, T.; Yi, D.; Hou, Y.; Wu, G. N-Acetylcysteine improves intestinal function and attenuates intestinal autophagy in piglets challenged with β-conglycinin. Sci. Rep. 2021, 11, 1261. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxid. Med. Cell Longev. 2019, 2019, 2843121. [Google Scholar] [CrossRef]
- Faria, A.; Monteiro, R.; Pestana, D.; Freitas, V.; Mateus, N.; Azevedo, I.; Calhau, C. Intestinal oxidative state can alter nutrient and drug bioavailability. Oxid. Med. Cell Longev. 2009, 2, 322–327. [Google Scholar] [CrossRef]
- Galla, R.; Grisenti, P.; Farghali, M.; Saccuman, L.; Ferraboschi, P.; Uberti, F. Ovotransferrin Supplementation Improves the Iron Absorption: An In Vitro Gastro-Intestinal Model. Biomedicines 2021, 9, 1543. [Google Scholar] [CrossRef]
- FDA. M9 Biopharmaceutics Classification System-Based Biowaivers. Available online: https://www.fda.gov/media/117974/download (accessed on 12 May 2021).
- EMA. ICH Guideline M9 on Biopharmaceutics Classification 5 System Based Biowaivers. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-m9-biopharmaceutics-classification-system-based-biowaivers-step-2b-first-version_en.pdf (accessed on 6 August 2018).
- Huang, C.H.; Shiu, S.M.; Wu, M.T.; Chen, W.L.; Wang, S.G.; Lee, H.M. Monacolin K affects lipid metabolism through SIRT1/AMPK pathway in HepG2 cells. Arch. Pharm. Res. 2013, 36, 1541–1551. [Google Scholar] [CrossRef]
- Frigerio, J.; Tedesco, E.; Benetti, F.; Insolia, V.; Nicotra, G.; Mezzasalma, V.; Pagliari, S.; Labra, M.; Campone, L. Anticholesterolemic Activity of Three Vegetal Extracts (Artichoke, Caigua, and Fenugreek) and Their Unique Blend. Front. Pharmacol. 2021, 12, 726199. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Abeysinghe, S.; Srisaisup, M.; Boonserm, P. Cytotoxic Effects and Intracellular Localization of Bin Toxin from. Toxins 2021, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef]
- Jogalekar, M.P.; Cooper, L.G.; Serrano, E.E. Hydrogel Environment Supports Cell Culture Expansion of a Grade IV Astrocytoma. Neurochem. Res. 2017, 42, 2610–2624. [Google Scholar] [CrossRef]
- Rayner, M.L.D.; Laranjeira, S.; Evans, R.E.; Shipley, R.J.; Healy, J.; Phillips, J.B. Developing an In Vitro Model to Screen Drugs for Nerve Regeneration. Anat. Rec. 2018, 301, 1628–1637. [Google Scholar] [CrossRef]
- Muscella, A.; Vetrugno, C.; Cossa, L.G.; Marsigliante, S. TGF-β1 activates RSC96 Schwann cells migration and invasion through MMP-2 and MMP-9 activities. J. Neurochem. 2020, 153, 525–538. [Google Scholar] [CrossRef]
- Ruga, S.; Galla, R.; Ferrari, S.; Invernizzi, M.; Uberti, F. Novel Approach to the Treatment of Neuropathic Pain Using a Combination with Palmitoylethanolamide and Equisetum arvense L. in an In Vitro Study. Int. J. Mol. Sci. 2023, 24, 5503. [Google Scholar] [CrossRef]
- Chua, P.; Lim, W.K. Optimisation of a PC12 cell-based in vitro stroke model for screening neuroprotective agents. Sci. Rep. 2021, 11, 8096. [Google Scholar] [CrossRef]
- Lahiani, A.; Brand-Yavin, A.; Yavin, E.; Lazarovici, P. Neuroprotective Effects of Bioactive Compounds and MAPK Pathway Modulation in “Ischemia”-Stressed PC12 Pheochromocytoma Cells. Brain Sci. 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Ruga, S.; Galla, R.; Penna, C.; Molinari, C.; Uberti, F. The Activity of Ten Natural Extracts Combined in a Unique Blend to Maintain Cholesterol Homeostasis—In Vitro Model. Int. J. Mol. Sci. 2022, 23, 3805. [Google Scholar] [CrossRef]
- Guha, S.; Alvarez, S.; Majumder, K. Transport of Dietary Anti-Inflammatory Peptide, γ-Glutamyl Valine (γ-EV), across the Intestinal Caco-2 Monolayer. Nutrients 2021, 13, 1448. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Galla, R.; Ruga, S.; Aprile, S.; Ferrari, S.; Brovero, A.; Grosa, G.; Molinari, C.; Uberti, F. New Hyaluronic Acid from Plant Origin to Improve Joint Protection—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 8114. [Google Scholar] [CrossRef]
- Kanmani, P.; Kim, H. Protective Effects of Lactic Acid Bacteria Against TLR4 Induced Inflammatory Response in Hepatoma HepG2 Cells Through Modulation of Toll-Like Receptor Negative Regulators of Mitogen-Activated Protein Kinase and NF-κB Signaling. Front. Immunol. 2018, 9, 1537. [Google Scholar] [CrossRef]
- Kulczar, C.; Lubin, K.E.; Lefebvre, S.; Miller, D.W.; Knipp, G.T. Development of a direct contact astrocyte-human cerebral microvessel endothelial cells blood-brain barrier coculture model. J. Pharm. Pharmacol. 2017, 69, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.C.; Wang, S.Y.; Chuang, Y.K.; Lee, W.Y.; Chang, W.C.; Wu, M.S.; Kuo, T.C.; Tsai, I.L. Clinical Mass Spectrometry Discovered Human IgG Sialylation as a Potential Biosignature for Kidney Function. J. Pers. Med. 2021, 11, 761. [Google Scholar] [CrossRef]
- Alexandrov, P.N.; Pogue, A.I.; Lukiw, W.J. Synergism in aluminum and mercury neurotoxicity. Integr. Food Nutr. Metab. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Bellinghausen, I.; Weigmann, B.; Zevallos, V.; Maxeiner, J.; Reißig, S.; Waisman, A.; Schuppan, D.; Saloga, J. Wheat amylase-trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice. J. Allergy Clin. Immunol. 2019, 143, 201–212.e204. [Google Scholar] [CrossRef]
- Morsanuto, V.; Galla, R.; Molinari, C.; Uberti, F. A New Palmitoylethanolamide Form Combined with Antioxidant Molecules to Improve Its Effectivess on Neuronal Aging. Brain Sci. 2020, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Petroni, D.; Tsai, J.; Agrawal, K.; Mondal, D.; George, W. Low-dose methylmercury-induced oxidative stress, cytotoxicity, and tau-hyperphosphorylation in human neuroblastoma (SH-SY5Y) cells. Environ. Toxicol. 2012, 27, 549–555. [Google Scholar] [CrossRef]
- Rzemieniec, J.; Litwa, E.; Wnuk, A.; Lason, W.; Gołas, A.; Krzeptowski, W.; Kajta, M. Neuroprotective action of raloxifene against hypoxia-induced damage in mouse hippocampal cells depends on ERα but not ERβ or GPR30 signalling. J. Steroid Biochem. Mol. Biol. 2015, 146, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudinezhad, M.; Abbaszadeh, F.; Zarezadeh, M.; Bahreini, N.; Jamilian, P.; Ostadrahimi, A. N-acetylecysteine, a powerful agent in the reinforcement of anti-oxidant profile: A systematic review and dose-response meta-analysis of controlled clinical trials. Clin. Nutr. ESPEN 2023, 54, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Megahed, A.; Gadalla, H.; Abdelhamid, F.M.; Almehmadi, S.J.; Khan, A.A.; Albukhari, T.A.; Risha, E.F. Vitamin D Ameliorates the Hepatic Oxidative Damage and Fibrotic Effect Caused by Thioacetamide in Rats. Biomedicines 2023, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef]
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The protective role of glutathione in osteoarthritis. J. Clin. Orthop. Trauma. 2021, 15, 145–151. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Marques, M.C.; Hacke, A.; Loubet Filho, P.S.; Cazarin, C.B.B.; Mariutti, L.R.B. Trust your gut: Bioavailability and bioaccessibility of dietary compounds. Curr. Res. Food Sci. 2022, 5, 228–233. [Google Scholar] [CrossRef]
- Holdiness, M.R. Clinical pharmacokinetics of N-acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Mazzei, L.; García Menéndez, S.; Martín Giménez, V.M.; Al Anouti, F.; Manucha, W. Genomic or Non-Genomic? A Question about the Pleiotropic Roles of Vitamin D in Inflammatory-Based Diseases. Nutrients 2023, 15, 767. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulè, S.; Ferrari, S.; Rosso, G.; Brovero, A.; Botta, M.; Congiusta, A.; Galla, R.; Molinari, C.; Uberti, F. The Combined Antioxidant Effects of N-Acetylcysteine, Vitamin D3, and Glutathione from the Intestinal–Neuronal In Vitro Model. Foods 2024, 13, 774. https://doi.org/10.3390/foods13050774
Mulè S, Ferrari S, Rosso G, Brovero A, Botta M, Congiusta A, Galla R, Molinari C, Uberti F. The Combined Antioxidant Effects of N-Acetylcysteine, Vitamin D3, and Glutathione from the Intestinal–Neuronal In Vitro Model. Foods. 2024; 13(5):774. https://doi.org/10.3390/foods13050774
Chicago/Turabian StyleMulè, Simone, Sara Ferrari, Giorgia Rosso, Arianna Brovero, Mattia Botta, Alessia Congiusta, Rebecca Galla, Claudio Molinari, and Francesca Uberti. 2024. "The Combined Antioxidant Effects of N-Acetylcysteine, Vitamin D3, and Glutathione from the Intestinal–Neuronal In Vitro Model" Foods 13, no. 5: 774. https://doi.org/10.3390/foods13050774