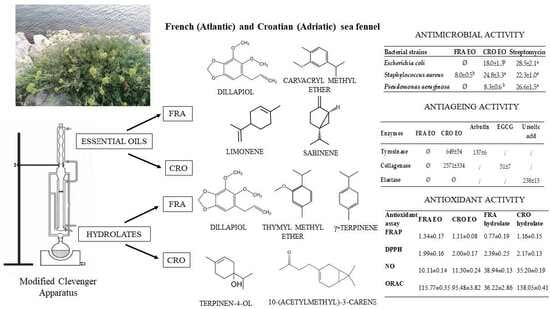
Volatiles from French and Croatian Sea Fennel Ecotypes: Chemical Profiles and the Antioxidant, Antimicrobial and Antiageing Activity of Essential Oils and Hydrolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Hydrodistillation
2.3. Chemical Analysis
2.4. Biological Activity
2.4.1. Antioxidant Activity
2.4.2. Antimicrobial Activity
2.4.3. Antiageing Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of EOs and Hydrolates
3.1.1. Essential Oils
3.1.2. Hydrolates
3.2. Biological Activities of EOs and Hydrolates
3.2.1. Antioxidant Activity
3.2.2. Antimicrobial Activity
3.2.3. Antiageing Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajeshwara Rao, B.R. Hydrosols and water-soluble essential oils: Medicinal and biological Properties. In Recent Progress in Medicinal Plants: Essential Oils I, 1st ed.; Studium Press LLC: Houston, TX, USA, 2013; Chapter 6; Volume 36. [Google Scholar]
- D’Amato, S.; Serio, A.; Chaves López, C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 18, 126–137. [Google Scholar] [CrossRef]
- Aćimović, M.; Tešević, V.; Smiljanic, K.; Cvetkovic, M.; Stankovic, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-Products of Essential Oil Distillation: Chemical Composition, Biological Activity and Potential Uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. Comptes Rendus Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef]
- De Santis, D.; Turchetti, G. Hydrolates: Characteristics, Properties, and Potential Uses in the Food Industry. In New Findings from Natural Substances, 1st ed.; Tiezzi, A., Ovidi, E., Karpiński, T.M., Eds.; Bentham Books: Sharjah, United Arab Emirates, 2022; Volume 28, pp. 183–210. [Google Scholar]
- Shafie, M.H.; Kamal, M.L.; Abdul Razak, N.A.; Hasan, S.; Uyup, N.H.; Rashid, N.F.A.; Zafarina, Z. Antioxidant and antimicrobial activity of plant hydrosol and Its Potential Application in Cosmeceutical Products. Jundishapur J. Nat. Pharm. Prod. 2022, 17, e124018. [Google Scholar] [CrossRef]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Hamedi, A.; Mahmoud Moheimani, S.; Sakhteman, S.; Etemadfard, H.; Moein, M. An overview on indications and chemical composition of aromatic waters (hydrosols) as functional beverages in Persian nutrition, culture and folk medicine for hyperlipidemia and cardiovascular conditions. J. Evid. Based Complement. Altern. Med. 2017, 22, 544–561. [Google Scholar] [CrossRef] [PubMed]
- Pedreiro, S.; Figueirinha, A.; Cavaleiro, C.; Cardoso, O.; Donato, M.M.; Salgueiro, L.; Ramos, F. Exploiting the Crithmum maritimum L. Aqueous Extracts and Essential Oil as Potential Preservatives in Food, Feed, Pharmaceutical and Cosmetic Industries. Antioxidants 2023, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R.; Billiani, F.; Singer, S.; Novak, J. Diversity of Essential Oils and the Respective Hydrolates Obtained from Three Pinus cembra Populations in the Austrian Alps. Appl. Sci. 2021, 11, 5686. [Google Scholar] [CrossRef]
- Atia, A.; Barhoumi, Z.; Mokded, R.; Abdelly, C.; Smaoui, A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plant Res. 2011, 5, 3564–3571. [Google Scholar]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, M.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crops Prod. 2020, 149, 112329. [Google Scholar] [CrossRef]
- Kraouia, M.; Nartea, A.; Maoloni, A.; Osimani, A.; Garofalo, C.; Fanesi, B.; Ismaiel, L.; Aquilanti, L.; Pacetti, D. Sea Fennel (Crithmum maritimum L.) as an Emerging Crop for the Manufacturing of Innovative Foods and Nutraceuticals. Molecules 2023, 28, 4741. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Antunes, M.; Tecelão, C.; Neves, M.; Pires, C.L.; Cruz, P.F.; Rodrigues, M.; Peralta, C.C.; Pereira, C.D.; Reboredo, F.; et al. Nutritive Value and Bioactivities of a Halophyte Edible Plant: Crithmum maritimum L. (Sea Fennel). Plants 2024, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Pateira, L.; Nogueira, T.; Antunes, A.; Venâncio, F.; Tavares, R.; Capelo, J. Two chemotypes of Crithmum maritimum L. from Portugal. Flavour Fragr. J. 1999, 14, 333–343. [Google Scholar] [CrossRef]
- Renna, M. Reviewing the Prospects of Sea Fennel (Crithmum maritimum L.) as Emerging Vegetable Crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Demirci, B.; Saritas, Y. Essential oil of Crithmum maritimum L. from Turkey. J. Essent. Oil Res. 2000, 12, 424–426. [Google Scholar] [CrossRef]
- Senatore, F.; Napolitano, F.; Özcan, M. Composition and antibacterial activity of the essential oil from Crithmum maritimum L. (Apiaceae) growing wild in Turkey. Flavour Fragr. J. 2000, 15, 186–189. [Google Scholar] [CrossRef]
- Özcan, M.; Akgül, A.; Başcr, K.H.C.; Özck, T.; Tabanca, N. Essential oil composition of sea fennel (Crithmum maritimum) form Turkey. Nahrung/Food 2001, 45, 353–356. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Pedro, L.G.; Cristina Figueiredo, A.; Barroso, J.G. Constituents of the essential oil of sea fennel (Crithmum maritimum L.) growing wild in Turkey. J. Med. Food 2006, 9, 128–130. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Uslu, N.; Figueredo, G.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Alsawmahi, O.N.; Özcan, M.M.; Ahmed, I.A.; Ahmed, M. The effect of fermentation process on bioactive properties, essential oil composition and phenolic constituents of raw fresh and fermented sea fennel (Crithmum maritimum L.) leaves. Indian J. Tradit. Knowl. 2019, 18, 800–804. [Google Scholar]
- Ruberto, G.; Tiziana Baratta, M.; Deans, S.G.; Damien Dorman, H.J. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oil. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Maggi, F.; Lupidi, F.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of sea fennel (Crithmum maritimum L., Apiaceae) essential oils against Culex quinquefasciatus Say and Spodoptera littoralis (Boisd.). Ind. Crops Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- D’Agostino, G.; Giambra, B.; Palla, F.; Bruno, M.; Badalamenti, N. The Application of the Essential Oils of Thymus vulgaris L. and Crithmum maritimum L. as Biocidal on Two Tholu Bommalu Indian Leather Puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Moreira, X.; Pérez-Ramos, I.M.; Matías, L.; Francisco, M.; García-González, A.; Martins-Noguerol, R.; Vázquez-González, C.; Abdala-Roberts, L.; Cambrollé, J. Efects of soil abiotic factors and plant chemical defences on seed predation on sea fennel (Crithmum maritimum). Plant Soil 2021, 465, 289–300. [Google Scholar] [CrossRef]
- Nabet, N.; Boudries, H.; Chougui, N.; Loupassaki, S.; Souagui, S.; Burló, F.; Hernàndez, F.; Carbonell-Barrachina, A.A.; Madani, K.; Larbat, R. Biological activities and secondary compound composition from Crithmum maritimum aerial parts. Int. J. Food Prop. 2017, 20, 1843–1855. [Google Scholar] [CrossRef]
- Zafeiropoulou, V.; Tomou, E.M.; Douros, A.; Skaltsa, H. The Effect of Successive Harvesting on The Volatile Constituents of Two Essential Oils of Cultivated Populations of Sea Fennel (Crithmum maritimum L.) in Greece. J. Essent. Oil-Bear. Plants 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Coiffard, L.; Piron-Frenet, M.; Amicel, L. Geographical variations of the constituents of the essential oil of Crithmum maritimum L., Apiaceae. Int. J. Cosmet. Sci. 1993, 15, 15–21. [Google Scholar] [CrossRef]
- Pereira, C.G.; Moraes, C.B.; Franco, C.H.; Feltrin, C.; Grougnet, R.; Barbosa, E.G.; Panciera, M.; Correia, C.R.D.; Rodrigues, M.J.; Custódio, L. In Vitro Anti-Trypanosoma cruzi Activity of Halophytes from Southern Portugal Reloaded: A Special Focus on Sea Fennel (Crithmum maritimum L.). Plants 2021, 10, 2235. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for delivery of Apiaceae essential oils—Towards highly effective and eco-friendly mosquito larvicides? Ind. Crops Prod 2019, 129, 631–640. [Google Scholar] [CrossRef]
- Bektasevic, M.; Jurin, M.; Roje, M.; Politeo, O. Phytochemical profile, antioxidant activity and cholinesterase inhibition potential of essential oil and extracts of Teucrium montanum from Bosnia and Herzegovina. Separations 2023, 10, 421. [Google Scholar] [CrossRef]
- Politeo, O.; Popović, M.; Veršić Bratinčević, M.; Koceić, P.; Ninčević Runjić, T.; Generalić Mekinić, I. Conventional vs. microwave-assisted hydrodistillation: Influence on the chemistry of sea fennel essential oil and its by-products. Plants 2023, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fuids and modifed version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Katalinić, V.; Smole Možina, S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Dorman, H.J.D.; Oinonen, P.P.; Darwis, Y.; Laakso, I.; Hiltunen, R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. Lebensmittel-Wissenschaft Technol. 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.D.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmac. 2008, 116, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for Tyrosinase Inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Kalyana Sundaram, I.; Deeptirekha Sarangi, D.; Sundararajan, V.; George, S.; Sheik Mohideen, S. Poly herbal formulation with anti-elastase and anti-oxidant properties for skin anti-aging. BMC Complement. Altern. Med. 2018, 18, 33. [Google Scholar] [CrossRef]
- Kulišić-Bilušić, T.; Blažević, I.; Dejanović, B.; Miloš, M.; Pifat, G. Evaluation of the antioxidant activity of essential oils from caper (Capparis Spinosa) and sea fennel (Crithmum Maritimum) by different methods. J. Food Biochem. 2010, 34, 286–302. [Google Scholar] [CrossRef]
- Generalić Meknić, I.; Blažević, I.; Mudnić, I.; Burčul, F.; Grga, M.; Skroza, D.; Jerčić, I.; Ljubenkov, I.; Boban, M.; Miloš, M.; et al. Sea fennel (Crithmum maritimum L.): Phytochemical profile, antioxidative, cholinesterase inhibitory and vasodilatory activity. J. Food Sci. Technol. 2016, 53, 3104–3112. [Google Scholar] [CrossRef]
- Politeo, O.; Popović, M.; Veršić Bratinčević, M.; Kovačević, K.; Urlić, B.; Generalić Mekinić, I. Chemical profiling of sea fennel (Crithmum maritimum L., Apiaceae) essential oils and their isolation residual waste-waters. Plants 2023, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Generalić Meknić, I.; Šimat, V.; Ljubenkov, I.; Burčul, F.; Grga, M.; Mihajlovski, M.; Lončar, R.; Katalinić, V.; Skroza, D. Influence of the vegetation period on sea fennel, Crithmum maritimum L. (Apiaceae), phenolic composition, antioxidant and anticholinesterase activities. Ind. Crop. Prod. 2018, 124, 947–953. [Google Scholar] [CrossRef]
- Inouye, S.; Takahashi, M.; Abe, S. A comparative study on the composition of fourty four hydrosols and their essential oils. Int. J. Essent. Oil Ther. 2008, 2, 89–104. [Google Scholar]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant hydrolates—Antioxidant properties, chemical composition and potential applications. Biomed. Pharmacother. 2021, 142, 112033. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components—an experimental and computational investigation. Nat Prod Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam Horm. 2005, 72, 505–535. [Google Scholar]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Hoskins, N.; Betts, G.; Mauriello, G. Changes in Membrane Fatty Acids Composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Lee, S.B.; Cha, K.H.; Kim, S.N.; Altantsetseg, S.; Shatar, S.; Sarangerel, O.; Nho, C.W. The antimicrobial activity of essential oil from Dracocephalum foetidum against pathogenic microorganisms. J. Microbiol. 2007, 45, 53–57. [Google Scholar]
- Celaya, L.S.; Alabrudzińska, M.H.; Molina, A.C.; Viturro, C.I.; Silvia, M. The Inhibition of Methicillin-Resistant Staphylococcus aureus by essential oils isolated from leaves and fruits of Schinus areira depending on their chemical compositions. Acta Biochim. Pol. 2014, 61, 41–46. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial activity and mechanism of limonene against Staphylococcus aureus. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, C.; Zhang, C.; Han, C.; Kuchkarova, N.; Shao, H. Chemical composition, phytotoxic, antimicrobial and insecticidal activity of the essential oils of Dracocephalum integrifolium. Toxins 2019, 11, 598. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Oboh, I.O. Peservatives|Traditional Preservatives—Vegetable Oils. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 137–140. [Google Scholar]
- De Almeida, R.R.P.; Souto, R.N.P.; Bastos, C.N.; da Silva, M.H.L.; Maia, J.G.S. Chemical Variation in Piper aduncum and biological properties of its dillapiole-rich essential oil. Chem. Biodivers. 2009, 6, 1427–1434. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Andrés-Juan, C.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization of Crithmum maritimum L. and daucus carota subsp. gummifer (syme) hook.fil. and their antimicrobial activity against apple tree and grapevine phytopathogens. Agronomy 2021, 11, 886. [Google Scholar] [CrossRef]
- Kunicka-Styczynska, A.; Smigielski, K.; Prusinowska, R.; Rajkowska, K.; Kusmider, B.; Sikora, M. Preservative activity of lavender hydrosols in moisturizing body gels. Lett. Appl. Microbiol. 2014, 60, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Kinetic studies of tyrosinase inhibitory activity of 19 essential oils extracted from endemic and exotic medicinal plants. S. Afr. J. Bot. 2016, 103, 89–94. [Google Scholar] [CrossRef]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Çol Ayvaz, M.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six algerian plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Lall, N.; Anina Lambrechts, I.; Reid, A.M.; Maphutha, J.; Nel, M.; Hassan, A.H.; Khalid, A.; Abdalla, A.N.; Van, B.L.; et al. In vitro and in silico pharmacological and cosmeceutical potential of ten essential oils from aromatic medicinal plants from the Mascarene Islands. Molecules 2022, 27, 8705. [Google Scholar] [CrossRef] [PubMed]

| RI | Compounds | FRA (%) | CRO (%) |
|---|---|---|---|
| 921 | α-thujene | 0.15 ± 0.02 | nd |
| 934 | α-pinene | 0.84 ± 0.13 | tr |
| 970 | Sabinene | 4.24 ± 0.10 | 51.47 ± 3.22 |
| 992 | β-pinene | 0.17 ± 0.02 | 0.89 ± 0.02 |
| 1012 | α-terpinene | 0.20 ± 0.02 | 0.98 ± 0.03 |
| 1020 | p-cymene | 2.97 ± 0.43 | tr |
| 1039 | (Z)-β-ocimene | 0.80 ± 0.17 | nd |
| 1032 | Limonene | nd | 36.28 ± 2.99 |
| 1042 | (E)-β-ocimene | nd | tr |
| 1056 | γ-terpinene | 9.88 ± 1.10 | 3.49 ± 0.07 |
| 1065 | cis-sabinene hydrate | nd | 0.10 ± 0.01 |
| 1086 | Terpinolene | 0.12 ± 0.01 | 0.37 ± 0.05 |
| 1118 | cis-p-menth-2-en-1-ol | nd | 0.10 ± 0.01 |
| 1176 | terpinen-4-ol | 0.25 ± 0.01 | 5.35 ± 0.04 |
| 1232 | thymyl methyl ether | 0.21 ± 0.01 | nd |
| 1242 | carvacryl methyl ether | 18.00 ± 2.40 | nd |
| 1620 | Dillapiole | 62.10 ± 1.83 | nd |
| TOTAL | 99.93 | 99.03 |
| RI | Compounds | FRA (%) | CRO (%) |
|---|---|---|---|
| 921 | α-thujene | 0.40 ± 0.02 | nd |
| 934 | α-pinene | 1.36 ± 0.02 | nd |
| 970 | sabinene | 5.64 ± 0.07 | nd |
| 986 | 2,3-dehydro-1,8-cineole | tr | nd |
| 989 | β-myrcene | 0.61 ± 0.07 | nd |
| 998 | octanal | tr | nd |
| 1001 | α-phellandrene | tr | nd |
| 1012 | α-terpinene | 0.62 ± 0.04 | nd |
| 1020 | p-cymene | 6.40 ± 0.07 | nd |
| 1024 | β-phellandrene | 0.19 ± 0.01 | nd |
| 1039 | (Z)-β-ocimene | 2.10 ±0.01 | nd |
| 1041 | benzeneacetaldehyde | 0.77 ± 0.15 | 5.06 ± 0.03 |
| 1056 | γ-terpinene | 9.34 ± 0.24 | nd |
| 1065 | cis-sabinene hydrate | tr | nd |
| 1086 | terpinolene | 0.35 ± 0.02 | nd |
| 1095 | trans-sabinene hydrate | tr | 4.73 ± 0.04 |
| 1097 | linalool | nd | tr |
| 1118 | cis-p-menth-2-en-1-ol | 0.25 ± 0.04 | 4.26 ± 0.02 |
| 1138 | trans-p-menth-2-en-1-ol | tr | 3.37 ± 0.05 |
| 1176 | terpinen-4-ol | 2.12 ± 0.22 | 41.93 ± 2.99 |
| 1183 | p-cymen-8-ol | tr | nd |
| 1188 | α-terpineol | 0.21 ± 0.01 | 5.71 ± 0.51 |
| 1205 | trans-pipertiol | nd | 2.46 ± 0.01 |
| 1218 | trans-carveol | 0.20 ± 0.01 | 3.36 ± 0.03 |
| 1222 | cis-carveol | nd | 3.75 ± 0.02 |
| 1232 | thymyl methyl ether | 26.30 ± 1.85 | nd |
| 1242 | carvacryl methyl ether | 0.28 ± 0.02 | nd |
| 1293 | thymol | 0.32 ± 0.03 | tr |
| 1301 | carvacrol | tr | 1.07 ± 0.02 |
| 1312 | p-vinylguaiacol | 0.76 ± 0.04 | nd |
| 1327 | myrtenyl acetate | nd | 0.99 ± 0.01 |
| 1384 | (E)-β-damascenone | nd | 1.03 ± 0.09 |
| 1390 | 10-(acetylmethyl)-3-carene | nd | 13.80 ± 2.01 |
| 1422 | dihydrodehydro-β-ionone | nd | 5.88 ± 0.09 |
| 1498 | bicyclogermacrene | 0.23 ± 0.01 | nd |
| 1521 | myristicin | 0.48 ± 0.00 | nd |
| 1557 | elemicin | tr | nd |
| 1563 | germacrene B | 0.39 ± 0.02 | nd |
| 1620 | dillapiole | 36.66 ± 5.66 | 1.52 ± 0.01 |
| TOTAL | 95.98 | 98.92 |
| Antioxidant Assay | FRA EO | CRO EO | FRA Hydrolate | CRO Hydrolate | Gallic Acid (Standard) |
|---|---|---|---|---|---|
| FRAP (µM Fe2+/L) | 1.34 ± 0.17 c | 1.11 ± 0.08 b | 0.77 ± 0.19 a | 1.16 ± 0.15 b | 2485.09 ± 3.73 * |
| DPPH (% inhibition) | 1.99 ± 0.16 a | 2.00 ± 0.17 a | 2.39 ± 0.25 b | 2.17 ± 0.13 ab | 95.35 ± 0.33 ** |
| NO (% inhibition) | 10.11 ± 0.14 a | 11.30 ± 0.24 b | 38.94 ± 0.13 d | 35.20 ± 0.19 c | 11.56 ± 0.90 ** |
| ORAC (µM Trolox equivalents/L) | 115.77 ± 0.35 bc | 95.48 ± 3.82 b | 36.22 ± 2.86 a | 138.05 ± 0.41 c | - |
| Bacterial Strains | FRA EO | CRO EO | FRA Hydrolate | CRO Hydrolate | Streptomycin |
|---|---|---|---|---|---|
| Escherichia coli | Ø | 18.0 ± 1.3 b | Ø | Ø | 28.5 ± 2.1 a |
| Staphylococcus aureus | 8.0 ± 0.5 b | 24.8 ± 3.3 a | Ø | Ø | 22.3 ± 1.0 a |
| Pseudomonas aeruginosa | Ø | 8.3 ± 0.6 b | Ø | Ø | 26.6 ± 1.5 a |
| Enzymes | FRA EO | CRO EO | Arbutin | EGCG | Ursolic Acid |
|---|---|---|---|---|---|
| Tyrosinase | Ø | 649 ± 54 | 137 ± 6 | / | / |
| Collagenase | Ø | 2571 ± 334 | / | 51 ± 7 | / |
| Elastase | Ø | Ø | / | / | 238 ± 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politeo, O.; Ćurlin, P.; Brzović, P.; Auzende, K.; Magné, C.; Generalić Mekinić, I. Volatiles from French and Croatian Sea Fennel Ecotypes: Chemical Profiles and the Antioxidant, Antimicrobial and Antiageing Activity of Essential Oils and Hydrolates. Foods 2024, 13, 695. https://doi.org/10.3390/foods13050695
Politeo O, Ćurlin P, Brzović P, Auzende K, Magné C, Generalić Mekinić I. Volatiles from French and Croatian Sea Fennel Ecotypes: Chemical Profiles and the Antioxidant, Antimicrobial and Antiageing Activity of Essential Oils and Hydrolates. Foods. 2024; 13(5):695. https://doi.org/10.3390/foods13050695
Chicago/Turabian StylePoliteo, Olivera, Pavao Ćurlin, Petra Brzović, Killian Auzende, Christian Magné, and Ivana Generalić Mekinić. 2024. "Volatiles from French and Croatian Sea Fennel Ecotypes: Chemical Profiles and the Antioxidant, Antimicrobial and Antiageing Activity of Essential Oils and Hydrolates" Foods 13, no. 5: 695. https://doi.org/10.3390/foods13050695