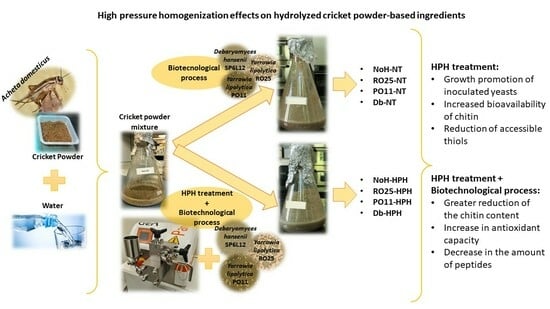
Effect of Combined High-Pressure Homogenization and Biotechnological Processes on Chitin, Protein, and Antioxidant Activity of Cricket Powder-Based Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions of Yeast Strains
2.2. Preparation of the Cricket Powder Hydrolysate and HPH Treatment
2.3. Microbiological Analysis and pH
2.4. Determination of Chitin Content
2.5. Protein Characterization
2.6. Total Accessible Thiols
2.7. In Vitro Total Antioxidant Capacity (TAC) Determination
2.8. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Quality and pH
3.2. Chitin Content
3.3. Protein Characterization
3.4. Thiolic Accessibility Characterization
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Ganguly, M.; Chandra, B.; Viswavidyalaya, K. ENTOMOPHAGY: Grab the Grub for a Better Future Agric. Food e-Newsl. 2019, 1, 11100. [Google Scholar]
- Dagevos, H. A Literature Review of Consumer Research on Edible Insects: Recent Evidence and New Vistas from 2019 Studies. J. Insects Food Feed 2020, 7, 249–259. [Google Scholar] [CrossRef]
- Pippinato, L.; Gasco, L.; Di Vita, G.; Mancuso, T. Current Scenario in the European Edible-Insect Industry: A Preliminary Study. J. Insects Food Feed. 2020, 6, 371–381. [Google Scholar] [CrossRef]
- Patrignani, F.; Parrotta, L.; Del Duca, S.; Vannini, L.; Camprini, L.; Dalla Rosa, M.; Schlüter, O.; Lanciotti, R. Potential of Yarrowia Lipolytica and Debaryomyces Hansenii Strains to Produce High Quality Food Ingredients Based on Cricket Powder. LWT 2020, 119, 108866. [Google Scholar] [CrossRef]
- Rossi, S.; Parrotta, L.; Del Duca, S.; Rosa, M.D.; Patrignani, F.; Schluter, O.; Lanciotti, R. Effect of Yarrowia Lipolytica RO25 Cricket-Based Hydrolysates on Sourdough Quality Parameters. LWT 2021, 148, 111760. [Google Scholar] [CrossRef]
- Rossi, S.; Parrotta, L.; Gottardi, D.; Glicerina, V.T.; Del Duca, S.; Rosa, M.D.; Patrignani, F.; Schlüter, O.; Lanciotti, R. Unravelling the Potential of Cricket-Based Hydrolysed Sourdough on the Quality of an Innovative Bakery Product. J. Insects Food Feed. 2022, 8, 921–935. [Google Scholar] [CrossRef]
- Psarianos, M.; Dimopoulos, G.; Ojha, S.; Cavini, A.C.M.; Bußler, S.; Taoukis, P.; Schlüter, O.K. Effect of Pulsed Electric Fields on Cricket (Acheta domesticus) Flour: Extraction Yield (Protein, Fat and Chitin) and Techno-Functional Properties. Innov. Food Sci. Emerg. 2022, 76, 102908. [Google Scholar] [CrossRef]
- Ugur, A.E.; Bolat, B.; Oztop, H.; Alpas, H. Effects of High Hydrostatic Pressure (HHP) Processing and Temperature on Physicochemical Characterization of Insect Oils Extracted from Acheta Domesticus (House Cricket) and Tenebrio Molitor (Yellow Mealworm). Waste Biomass Valorization 2021, 12, 4277–4286. [Google Scholar] [CrossRef]
- Patrignani, F.; Lanciotti, R. Applications of High and Ultra High Pressure Homogenization for Food Safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed]
- Tribst, L.; Ribeiro, L.R.; Cristianini, M. Comparison of the Effects of High Pressure Homogenization and High Pressure Processing on the Enzyme Activity and Antimicrobial Profile of Lysozyme. Innov. Food Sci. Emerg. Technol. 2017, 43, 60–67. [Google Scholar] [CrossRef]
- Navarro, J.L.; Izquierdo, L.; Carbonell, J.V.; Sentandreu, E. Effect of PH, Temperature and Maturity on Pectinmethylesterase Inactivation of Citrus Juices Treated by High-Pressure Homogenization. LWT 2014, 57, 785–788. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Rocculi, P.; Dalla Rosa, M. Strategies to Improve Food Functionality: Structure–Property Relationships on High Pressures Homogenization, Vacuum Impregnation and Drying Technologies. Trends Food Sci. Technol. 2015, 46, 1–12. [Google Scholar] [CrossRef]
- Moscovici Joubran, A.; Katz, I.H.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. The Effect of Pressure Level and Cycling in High-Pressure Homogenization on Physicochemical, Structural and Functional Properties of Filtered and Non-Filtered Strawberry Nectar. Innov. Food Sci. Emerg. 2019, 57, 102203. [Google Scholar] [CrossRef]
- Panozzo, A.; Manzocco, L.; Calligaris, S.; Bartolomeoli, I.; Maifreni, M.; Lippe, G.; Nicoli, M.C. Effect of High Pressure Homogenisation on Microbial Inactivation, Protein Structure and Functionality of Egg White. Food Res. Int. 2014, 62, 718–725. [Google Scholar] [CrossRef]
- Ono, Y.; Ogura, K.; Kaku, Y.; Fujisawa, S.; Isogai, A. Structural Changes in α-Chitin through Nanofibrillation by High-Pressure Homogenization in Water. Polym. J. 2020, 52, 813–818. [Google Scholar] [CrossRef]
- Satam, C.C.; Meredith, J.C. Increasing Efficiency of the Homogenization Process for Production of Chitin Nanofibers for Barrier Film Applications. Carbohydr. Polym. 2021, 274, 118658. [Google Scholar] [CrossRef]
- Davoudpour, Y.; Hossain, S.; Abdul Khalil, H.; Mohamad Haafiz, M.; Mohd Ishak, Z.; Hassan, A.; Islam Sarker, Z. Optimization of High Pressure Homogenization Parameters for the Isolation of Cellulosic Nanofibers Using Response Surface Methodology. Ind. Crop. Prod. 2015, 74, 381–387. [Google Scholar] [CrossRef]
- Zhai, M.; Du, J.; Zhang, J.; Miao, J.; Luo, J.; Cao, Y.; Duan, S. Changes in the Microstructure and Enzymatic Hydrolysis Performance of Chitin Treated by Steam Explosion, High-Pressure Homogenization, and γ Radiation. J. Appl. Polym. Sci. 2020, 137, 49597. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, A.; Chen, K.; Ouyang, P. Enzymatic Production of N-Acetyl-d-Glucosamine from Crayfish Shell Wastes Pretreated via High Pressure Homogenization. Carbohydr. Polym. 2017, 171, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Feng, X.; Li, C. Enhanced Production of β-Glucuronidase from Penicillium Purpurogenum Li-3 by Optimizing Fermentation and Downstream Processes. Front. Chem. Sci. Eng. 2015, 9, 501–510. [Google Scholar] [CrossRef]
- Nawawi, W.M.F.B.W.; Jones, M.; Murphy, R.J.; Lee, K.-Y.; Kontturi, E.; Bismarck, A. Nanomaterials Derived from Fungal Sources Is It the New Hype? Biomacromolecules 2019, 21, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Jeihanipour, A.; Edebo, L.; Nlklasson, C.; Taherzadeh, M.J. Determination of Glucosamine and N-Acetyl Glucosamine in Fungal Cell Walls. J. Agric. Food Chem. 2008, 56, 8314–8318. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Casiraghi, M.C.; Iametti, S.; Pagani, M.A.; Marti, A. Process Conditions Affect Starch Structure and Its Interactions with Proteins in Rice Pasta. Carbohydr. Polym. 2013, 92, 1865–1872. [Google Scholar] [CrossRef]
- Valli, V.; Gómez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar Cane and Sugar Beet Molasses, Antioxidant-Rich Alternatives to Refined Sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef]
- Maresca, P.; Donsì, F.; Ferrari, G. Application of a Multi-Pass High-Pressure Homogenization Treatment for the Pasteurization of Fruit Juices. J. Food Eng. 2011, 104, 364–372. [Google Scholar] [CrossRef]
- Roobab, U.; Shabbir, M.A.; Khan, A.W.; Arshad, R.N.; Bekhit, A.E.D.; Zeng, X.A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Treatments for Better Quality Clean-Label Juices and Beverages: Overview and Advances. LWT 2021, 149, 111828. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Gul, O.; Besir, A.; Atalar, I. Effect of High Pressure Homogenization (HPH) on Functional and Rheological Properties of Hazelnut Meal Proteins Obtained from Hazelnut Oil Industry by-Products. J. Food Eng. 2018, 233, 98–108. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Vannini, L.; Tossani, N.; Patrignani, F.; Lanciotti, R.; Bordoni, A. Impact of High Pressure Homogenization (HPH) Treatment on the Nutritional Quality of Egg/Yogurt, Vegetable and Fruit Based Creams. Food Nutr. Sci. 2014, 2014, 27–34. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Microbial, Nutritional, and Organoleptic Quality of Pomegranate Juice Following High-Pressure Homogenization and Low-Temperature Pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef]
- Pohling, J.; Hawboldt, K.; Dave, D. Comprehensive Review on Pre-Treatment of Native, Crystalline Chitin Using Non-Toxic and Mechanical Processes in Preparation for Biomaterial Applications. Green. Chem. 2022, 24, 6790–6809. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, K.; Girouard, N.; Meredith, J.C. Facile Route to Produce Chitin Nanofibers as Precursors for Flexible and Transparent Gas Barrier Materials. Biomacromolecules 2014, 15, 4614–4620. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia Lipolytica. Yeast 2012, 29, 409–418. [Google Scholar] [CrossRef]
- Breuer, U.; Harms, H. Debaryomyces Hansenii—An Extremophilic Yeast with Biotechnological Potential. Yeast 2006, 23, 415–437. [Google Scholar] [CrossRef]
- Carullo, D.; Donsì, F.; Ferrari, G. Influence of High-Pressure Homogenization on Structural Properties and Enzymatic Hydrolysis of Milk Proteins. LWT 2020, 130, 109657. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, M.; Shi, J.; Yang, B.; Li, J.; Luo, D.; Jiang, G.; Jiang, Y. Effects of Combined High-Pressure Homogenization and Enzymatic Treatment on Extraction Yield, Hydrolysis and Function Properties of Peanut Proteins. Innov. Food Sci. Emerg. Technol. 2011, 12, 478–483. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of Enzymatic Hydrolysis on Bioactive Properties and Allergenicity of Cricket (Gryllodes sigillatus) Protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K.K.; Merz, M.; Appel, D.; De Araujo, M.M.; Fischer, L. New Insights into the Flavoring Potential of Cricket (Acheta domesticus) and Mealworm (Tenebrio molitor) Protein Hydrolysates and Their Maillard Products. Food Chem. 2021, 364, 130336. [Google Scholar] [CrossRef]
- Santiago, L.A.; Fadel, O.M.; Tavares, G.M. How Does the Thermal-Aggregation Behavior of Black Cricket Protein Isolate Affect Its Foaming and Gelling Properties? Food Hydrocoll. 2021, 110, 106169. [Google Scholar] [CrossRef]
- Dion-Poulin, A.; Laroche, M.; Doyen, A.; Turgeon, S.L.; Kowalczewski, P.L.; Singh, A.P.; Kitts, D. Molecules Functionality of Cricket and Mealworm Hydrolysates Generated after Pretreatment of Meals with High Hydrostatic Pressures. Molecules 2020, 25, 5366. [Google Scholar] [CrossRef]
- Calligaris, S.; Gulotta, A.; Ignat, A.; Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V.; Nicoli, M.C. Milk Pre-Treatment by High Pressure Homogenization in the Manufacturing of “Queso Fresco” Fortified with Omega-3 Fatty Acids. LWT 2013, 50, 629–633. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Molecules Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Chen, J.; Chen, H.; Wu, Q.; Zhang, K.; Li, D.; Li, Y.; Chen, Y. Effects of High-Pressure Homogenization Extraction on the Physicochemical Properties and Antioxidant Activity of Large-Leaf Yellow Tea Polysaccharide Conjugates. Process Biochem. 2022, 122, 1359–5113. [Google Scholar] [CrossRef]
- Yong, S.X.M.; Song, C.P.; Choo, W.S. Impact of High-Pressure Homogenization on the Extractability and Stability of Phytochemicals. Front. Sustain. Food Syst. 2021, 4, 593259. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Manalo, M.; Yves Cheng Uy, L.; Ann Torio, M.O.; Mangussad, D.; Teresa Sucgang, A.; Clare Marie Clemencia, M.; Yves Uy, L.; Ann Torio, M. Isolation and Characterization of the Total Protein in “Lakatan” Banana (Musa acuminata Colla) with Bioactive Peptides Exhibiting Antioxidative and Antihypertensive Activities. Philipp. Agric. Scientist 2021, 104, 268–277. [Google Scholar]
- Liu, Y.; Wan, S.; Liu, J.; Zou, Y.; Liao, S. Antioxidant activity and stability study of peptides from enzymatically hydrolyzed male silkmoth. J. Food Process 2017, 41, e13081. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Xie, M.Y. High Pressure Homogenization Increases Antioxidant Capacity and Short-Chain Fatty Acid Yield of Polysaccharide from Seeds of Plantago asiatica L. Food Chem. 2013, 138, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Luo, Z.C.; Yuan, F.; Yu, X. bin Combined Process of High-Pressure Homogenization and Hydrothermal Extraction for the Extraction of Fucoidan with Good Antioxidant Properties from Nemacystus Decipients. Food Bioprod. Process 2017, 106, 35–42. [Google Scholar] [CrossRef]
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules 2021, 26, 1802. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, S.; Gottardi, D.; Barbiroli, A.; Di Nunzio, M.; Siroli, L.; Braschi, G.; Schlüter, O.; Patrignani, F.; Lanciotti, R. Effect of Combined High-Pressure Homogenization and Biotechnological Processes on Chitin, Protein, and Antioxidant Activity of Cricket Powder-Based Ingredients. Foods 2024, 13, 449. https://doi.org/10.3390/foods13030449
Rossi S, Gottardi D, Barbiroli A, Di Nunzio M, Siroli L, Braschi G, Schlüter O, Patrignani F, Lanciotti R. Effect of Combined High-Pressure Homogenization and Biotechnological Processes on Chitin, Protein, and Antioxidant Activity of Cricket Powder-Based Ingredients. Foods. 2024; 13(3):449. https://doi.org/10.3390/foods13030449
Chicago/Turabian StyleRossi, Samantha, Davide Gottardi, Alberto Barbiroli, Mattia Di Nunzio, Lorenzo Siroli, Giacomo Braschi, Oliver Schlüter, Francesca Patrignani, and Rosalba Lanciotti. 2024. "Effect of Combined High-Pressure Homogenization and Biotechnological Processes on Chitin, Protein, and Antioxidant Activity of Cricket Powder-Based Ingredients" Foods 13, no. 3: 449. https://doi.org/10.3390/foods13030449