Effects of Roasting Temperatures on Peanut Oil and Protein Yield Extracted via Aqueous Enzymatic Extraction and Stability of the Oil Body Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
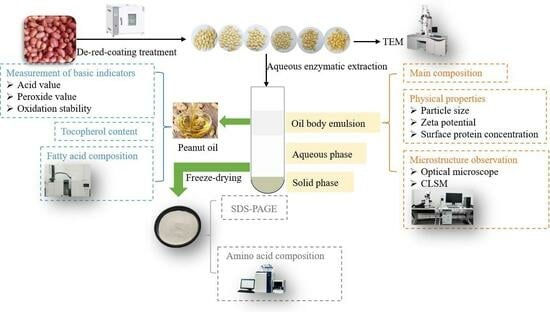
2.2. Aqueous Enzymatic Extraction of Peanut Oil and Protein
2.3. Analysis of the Main Composition of OBE
2.4. Physical Properties
2.4.1. Determination of Particle Size Distribution and Zeta Potential
2.4.2. Measurement of Surface Protein Concentration
2.5. Microstructure Observation
2.5.1. Optical Microscopy
2.5.2. Confocal Laser Scanning Microscopy (CLSM)
2.5.3. Transmission Electron Microscopy (TEM)
2.6. Evaluation of Oil Quality
2.6.1. Measurement of Basic Indicators
2.6.2. Determination of Tocopherol Content
2.6.3. Determination of Fatty Acid Composition
2.7. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8. Determination of Amino Acid Composition
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Roasting Temperatures on the Extraction Rate of Peanut Oil and Protein
3.2. Effect of Roasting Temperatures on the Composition of OBE
3.3. Effect of Roasting Temperature on the Stability of Peanut OBEs
3.4. Microstructure of OBEs
3.5. Effect of Different Roasting Temperatures on Peanut Oil Quality
3.5.1. Analysis of Physicochemical Indicators and Tocopherol Content
3.5.2. Analysis of Fatty Acid Composition
3.6. Effect of Different Roasting Temperatures on Protein Quality
3.6.1. Effect of Different Roasting Temperatures on Polypeptide Composition
3.6.2. Effect of Different Roasting Temperatures on Amino Acid Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/advQuery (accessed on 15 September 2023).
- Niu, R.H.; Chen, F.S.; Liu, C.; Duan, X.J. Composition and Rheological Properties of Peanut Oil Bodies from Aqueous Enzymatic Extraction. J. Oleo Sci. 2021, 70, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, F.; Assaf, J.C.; Elias, R.; Debs, E.; Louka, N. Defatting and Defatted Peanuts: A Critical Review on Methods of Oil Extraction and Consideration of Solid Matrix as a By-Product or Intended Target. Processes 2023, 11, 2512. [Google Scholar] [CrossRef]
- Liu, C.; Chen, F.S.; Xia, Y.M.; Liu, B.Y. Physicochemical and rheological properties of peanut oil body following alkaline pH treatment. LWT-Food Sci. Technol. 2022, 154, 112590. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Gordon, M.H.; Ezeh, O.; Niranjan, K. Aqueous enzymatic extraction of Moringa oleifera oil. Food Chem. 2016, 211, 400–408. [Google Scholar] [CrossRef]
- Shrestha, K.; De Meulenaer, B. Effect of Seed Roasting on Canolol, Tocopherol, and Phospholipid Contents, Maillard Type Reactions, and Oxidative Stability of Mustard and Rapeseed Oils. J. Agric. Food Chem. 2014, 62, 5412–5419. [Google Scholar] [CrossRef]
- Wijesundera, C.; Ceccato, C.; Fagan, P.; Shen, Z.P. Seed roasting improves the oxidative stability of canola (B-napus) and mustard (B-juncea) seed oils. Eur. J. Lipid Sci. Technol. 2008, 110, 360–367. [Google Scholar] [CrossRef]
- Li, P.F.; Gasmalla, M.A.A.; Zhang, W.B.; Liu, J.J.; Bing, R.; Yang, R.J. Effects of roasting temperatures and grinding type on the yields of oil and protein obtained by aqueous extraction processing. J. Food Eng. 2016, 173, 15–24. [Google Scholar] [CrossRef]
- Huang, A.H.C. Plant Lipid Droplets and Their Associated Proteins: Potential for Rapid Advances. Plant Physiol. 2018, 176, 1894–1918. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Siniossoglou, S. Function of lipid droplet-organelle interactions in lipid homeostasis. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1459–1468. [Google Scholar] [CrossRef]
- Hu, M.; Du, X.Q.; Liu, G.N.; Tan, Z.; Zhang, S.; Qi, B.K.; Li, Y. Investigation of structure-stability correlations of reconstructed oil bodies. LWT-Food Sci. Technol. 2022, 165, 113740. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zheng, Y.Z.; Yao, F.; Chen, F.S. Effects of pH and temperature on the stability of peanut oil bodies: New insights for embedding active ingredients. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 654, 130110. [Google Scholar] [CrossRef]
- Zaaboul, F.; Raza, H.; Chen, C.; Liu, Y.F. Characterization of Peanut Oil Bodies Integral Proteins, Lipids, and Their Associated Phytochemicals. J. Food Sci. 2018, 83, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zaaboul, F.; Zhao, Q.L.; Xu, Y.J.; Liu, Y.F. Soybean oil bodies: A review on composition, properties, food applications, and future research aspects. Food Hydrocoll. 2022, 124, 107296. [Google Scholar] [CrossRef]
- Zhao, L.P.; Chen, Y.M.; Chen, Y.J.; Kong, X.Z.; Hua, Y.F. Effects of pH on protein components of extracted oil bodies from diverse plant seeds and endogenous protease-induced oleosin hydrolysis. Food Chem. 2016, 200, 125–133. [Google Scholar] [CrossRef]
- Liu, C.; Chen, F.S.; Xia, Y.M. Composition and structural characterization of peanut crude oil bodies extracted by aqueous enzymatic method. J. Food Compos. Anal. 2022, 105, 104238. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Chen, F.S.; Hao, L.H.; Du, Y.; Liu, C. Peanut Oil Body Composition and Stability. J. Food Sci. 2019, 84, 2812–2819. [Google Scholar] [CrossRef]
- Liu, C.; Chen, F.S.; Niu, R.H.; Gao, Y.H. Effects of Pretreatment on the Yield of Peanut Oil and Protein Extracted by Aqueous Enzymatic Extraction and the Characteristics of the Emulsion. J. Oleo Sci. 2020, 69, 1445–1453. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Chen, F.S.; Wang, Y.Y. Demulsification of peanut emulsion by aqueous enzymatic extraction using a combination of oleic and citric acids. LWT-Food Sci. Technol. 2023, 180, 114658. [Google Scholar] [CrossRef]
- Xu, D.X.; Gao, Q.R.; Ma, N.N.; Hao, J.; Yuan, Y.M.; Zhang, M.; Cao, Y.P.; Ho, C.T. Structures and physicochemical characterization of enzyme extracted oil bodies from rice bran. LWT-Food Sci. Technol. 2021, 135, 109982. [Google Scholar] [CrossRef]
- Chabrand, R.M.; Kim, H.J.; Zhang, C.; Glatz, C.E.; Jung, S. Destabilization of the emulsion formed during aqueous extraction of soybean oil. J. Am. Oil Chem. Soc. 2008, 85, 383–390. [Google Scholar] [CrossRef]
- Li, P.F.; Gasmalla, M.A.A.; Liu, J.J.; Zhang, W.B.; Yang, R.J.; Aboagarib, E.A.A. Characterization and demusification of cream emulsion from aqueous extraction of peanut. J. Food Eng. 2016, 185, 62–71. [Google Scholar] [CrossRef]
- Cao, J.B.; Song, Y.T.; Wu, H.; Qin, L.H.; Hu, L.H.; Hao, R. Ultrastructural Studies on the Natural Leaf Senescence of Cinnamomum Camphora. Scanning 2013, 35, 336–343. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official Methods and Recommended Practices ofthe American Oil Chemists’ Society; Association of Official Analytical Chemists: Arlington, VA, USA, 2009. [Google Scholar]
- Gao, Y.H.; Zheng, Y.Z.; Yao, F.; Chen, F.S. A Novel Strategy for the Demulsification of Peanut Oil Body by Caproic Acid. Foods 2023, 12, 3029. [Google Scholar] [CrossRef]
- Ji, J.M.; Liu, Y.L.; Shi, L.K.; Wang, N.N.; Wang, X.D. Effect of roasting treatment on the chemical composition of sesame oil. LWT-Food Sci. Technol. 2019, 101, 191–200. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Chen, F.S.; Liu, K.L.; Zhu, T.W.; Jiang, L.Z. Combination of Alcalase 2.4 L and CaCl2 for aqueous extraction of peanut oil. J. Food Sci. 2020, 85, 1772–1780. [Google Scholar] [CrossRef]
- Liu, K.L.; Liu, Y.; Chen, F.S. Effect of gamma irradiation on the physicochemical properties and nutrient contents of peanut. Lwt-Food Sci. Technol. 2018, 96, 535–542. [Google Scholar] [CrossRef]
- Vovk, H.; Karnpakdee, K.; Ludwig, R.; Nosenko, T. Enzymatic Pretreatment of Plant Cells for Oil Extraction. Food Technol. Biotechnol. 2023, 61, 160–178. [Google Scholar] [CrossRef]
- Yang, J.C.; Vardar, U.S.; Boom, R.M.; Bitter, J.H.; Nikiforidis, C.V. Extraction of oleosome and protein mixtures from sunflower seeds. Food Hydrocoll. 2023, 145, 109078. [Google Scholar] [CrossRef]
- Nikiforidis, C.V. Structure and functions of oleosomes (oil bodies). Adv. Colloid Interface Sci. 2019, 274, 102039. [Google Scholar] [CrossRef]
- Zhao, L.P.; Chen, Y.M.; Yan, Z.H.; Kong, X.Z.; Hua, Y.F. Physicochemical and rheological properties and oxidative stability of oil bodies recovered from soybean aqueous extract at different pHs. Food Hydrocoll. 2016, 61, 685–694. [Google Scholar] [CrossRef]
- Zhang, S.B.; Lu, Q.Y. Characterizing the structural and surface properties of proteins isolated before and after enzymatic demulsification of the aqueous extract emulsion of peanut seeds. Food Hydrocoll. 2015, 47, 51–60. [Google Scholar] [CrossRef]
- Barros, M.; Fleuri, L.F.; Macedo, G.A. Seed Lipases: Sources, Applications And Properties—A Review. Braz. J. Chem. Eng. 2010, 27, 15–29. [Google Scholar] [CrossRef]
- Tzen, J.T.C.; Cao, Y.; Laurent, P.; Ratnayake, C.; Huang, A.H.C. Lipids, Proteins, and Structure of Seed Oil Bodies from Diverse Species. Plant Physiol. 1993, 101, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Castellani, O.; Belhomme, C.; David-Briand, E.; Guerin-Dubiard, C.; Anton, M. Oil-in-water emulsion properties and interfacial characteristics of hen egg yolk phosvitin. Food Hydrocoll. 2006, 20, 35–43. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Sidzhakova, D.; Ivanov, I.B.; Campbell, B. Interrelation between drop size and protein adsorption at various emulsification conditions. Langmuir 2003, 19, 5640–5649. [Google Scholar] [CrossRef]
- Liu, B.G.; Du, J.Q.; Zeng, J.; Chen, C.G.; Niu, S.Y. Characterization and antioxidant activity of dihydromyricetin-lecithin complex. Eur. Food Res. Technol. 2009, 230, 325–331. [Google Scholar] [CrossRef]
- Gao, P.; Cao, Y.; Liu, R.J.; Jin, Q.Z.; Wang, X.G. Phytochemical Content, Minor-Constituent Compositions, and Antioxidant Capacity of Screw-Pressed Walnut Oil Obtained from Roasted Kernels. Eur. J. Lipid Sci. Technol. 2019, 121, 1700229. [Google Scholar] [CrossRef]
- Ji, J.M.; Zhang, Y.; Wang, Y.; Wang, D.; Jie, H. Influence of seed-roasting degree on quality attributes of sunflower oil. J. Food Sci. 2023, 88, 4028–4045. [Google Scholar] [CrossRef]
- Davis, J.P.; Dean, L.L.; Price, K.M.; Sanders, T.H. Roast effects on the hydrophilic and lipophilic antioxidant capacities of peanut flours, blanched peanut seed and peanut skins. Food Chem. 2010, 119, 539–547. [Google Scholar] [CrossRef]
- Li, K.S.; Ali, M.A.; Muhammad, I.I.; Othman, N.H.; Noor, A.M. The Effect of Microwave Roasting Over the Thermooxidative Degradation of Perah Seed Oil During Heating. J. Oleo Sci. 2018, 67, 497–505. [Google Scholar] [CrossRef]
- Fathi-Achachlouei, B.; Azadmard-Damirchi, S.; Zahedi, Y.; Shaddel, R. Microwave pretreatment as a promising strategy for increment of nutraceutical content and extraction yield of oil from milk thistle seed. Ind. Crops Prod. 2019, 128, 527–533. [Google Scholar] [CrossRef]
- Bykova, O.; Shevkunov, O.; Kostyunina, O. Overview of SNPs Associated with Trans Fat Content in Cow’s Milk. Agriculture 2023, 13, 1151. [Google Scholar] [CrossRef]
- Capellini, M.C.; Giacomini, V.; Cuevas, M.S.; Rodrigues, C.E.C. Rice bran oil extraction using alcoholic solvents: Physicochemical characterization of oil and protein fraction functionality (vol 104, pg 133, 2017). Ind. Crops Prod. 2018, 123, 807. [Google Scholar] [CrossRef]







| Constituent | Pressed Oil | Leaching Oil | Commercial Oil | AEE | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 70 °C | 90 °C | 110 °C | 130 °C | 150 °C | ||||
| Acid value/(mg KOH/g) | 1.33 ± 0.01 a | 0.12 ± 0.007 e | 0.58 ± 0.02 b | 0.18 ± 0.005 d | 0.22 ± 0.03 d | 0.22 ± 0.02 d | 0.2 ± 0.03 d | 0.2 ± 0.02 d | 0.35 ± 0.02 c |
| Peroxide value/(g/100 g) | 0.19 ± 0.002 a | 0.03 ± 0.002 d | 0.1 ± 0.005 b | 0.12 ± 0.002 b | 0.07 ± 0.002 c | 0.09 ± 0.002 b | 0.1 ± 0.007 b | 0.09 ± 0.002 b | 0.06 ± 0.002 c |
| Oxidation stability/(h) | 2.44 ± 0.05 c | 4.46 ± 0.02 a | 3.25 ± 0.08 b | 0.88 ± 0.04 g | 0.63 ± 0.01 h | 0.86 ± 0.06 g | 2.2 ± 0.03 d | 1.37 ± 0.01 f | 1.8 ± 0.06 e |
| Tocopherols (mg/100 g) | |||||||||
| α | 10.51 ± 0.23 c | 10.88 ± 0.48 bc | 10.74 ± 0.03 bc | 11.49 ± 0.13 a | 11.17 ± 0.04 ab | 10.97 ± 0.04 bc | 10.78 ± 0.08 bc | 10.59 ± 0.03 c | 9.86 ± 0.21 d |
| γ | 12.46 ± 0.16 d | 12.59 ± 0.04 cd | 12.46 ± 0.30 d | 13.22 ± 0.14 a | 12.96 ± 0.03 ab | 13.01 ± 0.10 ab | 12.96 ± 0.16 ab | 12.86 ± 0.03 bc | 12.96 ± 0.11 ab |
| δ | 0.98 ± 0.06 a | 1.02 ± 0.07 a | 1.00 ± 0.16 a | 1.03 ± 0.03 a | 1.00 ± 0.04 a | 1.06 ± 0.03 a | 0.98 ± 0.13 a | 0.87 ± 0.01 a | 1.00 ± 0.01 a |
| Total contents | 23.48 ± 0.41 cd | 24.00 ± 0.56 bcd | 23.71 ± 0.41 cd | 25.24 ± 0.29 a | 24.69 ± 0.09 ab | 24.56 ± 0.16 ab | 24.25 ± 0.31 bc | 23.90 ± 0.06 bcd | 23.36 ± 0.33 d |
| Fatty Acid | Pressed Oil | Leaching Oil | Commercial Oil | AEE | |||||
|---|---|---|---|---|---|---|---|---|---|
| 50 °C | 70 °C | 90 °C | 110 °C | 130 °C | 150 °C | ||||
| C14:0 | 0.03 ± 0.00 c | 0.19 ± 0.00 a | 0.04 ± 0.00 b | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.00 c |
| C16:0 | 12.56 ± 0.20 c | 12.36 ± 0.02 c | 9.89 ± 0.02 e | 12.78 ± 0.01 b | 13.29 ± 0.01 a | 13.08 ± 0.01 a | 12.41 ± 0.01 c | 12.03 ± 0.01 d | 12.10 ± 0.00 d |
| C16:1 | 0.09 ± 0.00 a | 0.04 ± 0.00 c | 0.06 ± 0.00 b | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.04 ± 0.00 c | 0.05 ± 0.00 c | 0.04 ± 0.00 c | 0.04 ± 0.00 c |
| C17:0 | 0.07 ± 0.01 a | 0.04 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b | 0.05 ± 0.00 b |
| C18:0 | 4.13 ± 0.04 a | 3.93 ± 0.01 b | 3.58 ± 0.00 d | 4.06 ± 0.02 a | 4.09 ± 0.00 a | 4.13 ± 0.00 a | 3.87 ± 0.00 b | 3.74 ± 0.00 c | 3.81 ± 0.01 b |
| CT18:1 | - | - | 0.04 ± 0.00 a | - | - | - | - | - | - |
| C18:1 | 50.60 ± 0.00 a | 38.93 ± 0.03 d | 48.88 ± 0.00 b | 39.00 ± 0.07 c | 39.02 ± 0.03 c | 38.31 ± 0.02 e | 37.92 ± 0.03 f | 37.37 ± 0.03 h | 37.60 ± 0.01 g |
| CT18:2 | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.12 ± 0.00 a | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.00 b |
| C18:2 | 25.21 ± 0.27 d | 39.20 ± 0.05 a | 31.71 ± 0.02 c | 37.76 ± 0.05 b | 37.28 ± 0.03 b | 38.31 ± 0.03 b | 39.89 ± 0.01 a | 41.05 ± 0.02 a | 40.79 ± 0.03 a |
| C20:0 | 1.62 ± 0.02 a | 1.44 ± 0.05 c | 1.26 ± 0.01 d | 1.62 ± 0.00 a | 1.62 ± 0.01 a | 1.61 ± 0.00 a | 1.54 ± 0.00 b | 1.49 ± 0.02 c | 1.48 ± 0.01 c |
| C20:1 | 0.92 ± 0.00 a | 0.70 ± 0.01 b | 0.91 ± 0.02 a | 0.78 ± 0.00 b | 0.76 ± 0.00 b | 0.75 ± 0.01 b | 0.74 ± 0.00 b | 0.75 ± 0.01 b | 0.72 ± 0.01 b |
| C22:0 | 3.22 ± 0.03 a | 2.11 ± 0.03 c | 2.40 ± 0.02 b | 2.42 ± 0.00 b | 2.41 ± 0.00 b | 2.39 ± 0.01 b | 2.29 ± 0.00 b | 2.25 ± 0.01 bc | 2.22 ± 0.00 c |
| C20:3 | 0.08 ± 0.00 a | - | - | - | - | - | - | - | - |
| C24:0 | 1.44 ± 0.00 a | 1.02 ± 0.02 d | 1.06 ± 0.01 cd | 1.42 ± 0.09 a | 1.39 ± 0.07 ab | 1.30 ± 0.03 b | 1.18 ± 0.02 c | 1.16 ± 0.02 c | 1.13 ± 0.01 c |
| MUFA | 51.61 ± 0.01 a | 39.67 ± 0.02 c | 49.85 ± 0.02 b | 39.82 ± 0.07 c | 39.82 ± 0.03 c | 39.09 ± 0.00 d | 38.71 ± 0.02 e | 38.16 ± 0.02 e | 38.36 ± 0.00 e |
| PUFA | 25.29 ± 0.27 f | 39.20 ± 0.05 c | 31.71 ± 0.02 e | 37.76 ± 0.05 d | 37.28 ± 0.03 d | 38.31 ± 0.03 d | 39.89 ± 0.01 c | 41.05 ± 0.02 a | 40.79 ± 0.03 b |
| UFA | 76.90 ± 0.28 e | 78.87 ± 0.07 c | 81.56 ± 0.00 a | 77.58 ± 0.11 d | 77.09 ± 0.06 e | 77.40 ± 0.03 d | 78.60 ± 0.04 c | 79.21 ± 0.04 b | 79.15 ± 0.02 b |
| SFA | 23.08 ± 0.28 a | 21.10 ± 0.07 c | 18.28 ± 0.00 d | 22.39 ± 0.11 b | 22.88 ± 0.06 a | 22.58 ± 0.03 b | 21.38 ± 0.04 c | 20.76 ± 0.04 c | 20.83 ± 0.02 c |
| O/L | 2.01 ± 0.02 a | 0.99 ± 0.00 c | 1.54 ± 0.00 b | 1.03 ± 0.00 c | 1.05 ± 0.00 c | 1.00 ± 0.00 c | 0.95 ± 0.00 d | 0.91 ± 0.00 d | 0.92 ± 0.00 d |
| Amino Acid | Roast Temperature (°C) | |||||
|---|---|---|---|---|---|---|
| 50 | 70 | 90 | 110 | 130 | 150 | |
| Asp | 12.64 ± 0.09 ab | 12.87 ± 0.04 a | 12.75 ± 0.34 a | 12.42 ± 0.30 ab | 12.40 ± 0.46 ab | 12.08 ± 0.01 b |
| Thr | 2.57 ± 0.44 b | 3.26 ± 0.01 a | 3.03 ± 0.15 ab | 3.02 ± 0.04 ab | 2.92 ± 0.05 ab | 2.97 ± 0.04 ab |
| Ser | 6.36 ± 0.38 c | 6.91 ± 0.03 ab | 7.08 ± 0.10 a | 6.66 ± 0.01 abc | 6.55 ± 0.04 bc | 6.56 ± 0.08 bc |
| Glu | 21.16 ± 0.26 a | 20.92 ± 0.12 a | 20.92 ± 0.21 a | 20.74 ± 0.34 a | 20.79 ± 0.23 a | 21.24 ± 0.04 a |
| Gly | 8.34 ± 0.07 ab | 8.09 ± 0.07 ab | 8.00 ± 0.13 b | 8.12 ± 0.23 ab | 7.94 ± 0.38 b | 8.48 ± 0.02 a |
| Ala | 5.67 ± 0.20 a | 5.52 ± 0.08 ab | 5.60 ± 0.15 ab | 5.51 ± 0.29 ab | 5.48 ± 0.23 ab | 5.21 ± 0.01 b |
| Cys | 0.67 ± 0.31 a | 0.81 ± 0.08 a | 0.78 ± 0.02 a | 0.68 ± 0.02 a | 0.68 ± 0.03 a | 0.90 ± 0.00 a |
| Val | 5.02 ± 0.37 ab | 5.06 ± 0.04 a | 5.11 ± 0.01 a | 4.95 ± 0.15 ab | 4.88 ± 0.15 ab | 4.53 ± 0.04 b |
| Met | 2.24 ± 0.07 ab | 2.22 ± 0.11 b | 2.05 ± 0.08 b | 2.49 ± 0.65 ab | 2.67 ± 0.87 ab | 3.25 ± 0.10 a |
| Ile | 3.97 ± 0.04 a | 3.91 ± 0.02 a | 3.93 ± 0.03 a | 3.94 ± 0.14 a | 3.99 ± 0.13 a | 3.83 ± 0.02 a |
| Leu | 7.69 ± 0.01 a | 7.49 ± 0.03 a | 7.59 ± 0.00 a | 7.75 ± 0.26 a | 7.93 ± 0.45 a | 7.75 ± 0.04 a |
| Tyr | 3.20 ± 0.04 b | 3.15 ± 0.02 b | 3.20 ± 0.03 b | 3.47 ± 0.18 a | 3.46 ± 0.13 a | 3.54 ± 0.01 a |
| Phe | 4.72 ± 0.04 ab | 4.61 ± 0.03 b | 4.66 ± 0.00 b | 5.12 ± 0.49 ab | 5.32 ± 0.44 a | 5.01 ± 0.06 ab |
| His | 2.59 ± 0.06 a | 2.50 ± 0.09 a | 2.52 ± 0.01 a | 2.57 ± 0.15 a | 2.63 ± 0.12 a | 2.65 ± 0.00 a |
| Lys | 3.50 ± 0.05 a | 3.38 ± 0.02 a | 3.25 ± 0.01 b | 3.09 ± 0.10 c | 2.89 ± 0.09 d | 2.76 ± 0.00 e |
| Arg | 9.32 ± 0.11 a | 8.97 ± 0.13 a | 9.21 ± 0.07 a | 9.02 ± 0.43 a | 9.02 ± 0.45 a | 8.83 ± 0.03 a |
| Pro | 0.31 ± 0.13 a | 0.33 ± 0.01 a | 0.35 ± 0.19 a | 0.45 ± 0.01 a | 0.45 ± 0.02 a | 0.42 ± 0.00 a |
| EAA | 29.72 ± 0.13 a | 29.93 ± 0.13 a | 29.60 ± 0.26 a | 30.35 ± 1.26 a | 30.60 ± 1.59 a | 30.09 ± 0.11 a |
| HAA | 29.63 ± 0.07 a | 29.13 ± 0.07 a | 29.28 ± 0.45 a | 30.22 ± 1.11 a | 31.92 ± 3.18 a | 29.98 ± 0.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, Y.; Liu, C.; Chen, F.; Yin, L. Effects of Roasting Temperatures on Peanut Oil and Protein Yield Extracted via Aqueous Enzymatic Extraction and Stability of the Oil Body Emulsion. Foods 2023, 12, 4183. https://doi.org/10.3390/foods12224183
Zhang Y, Chen Y, Liu C, Chen F, Yin L. Effects of Roasting Temperatures on Peanut Oil and Protein Yield Extracted via Aqueous Enzymatic Extraction and Stability of the Oil Body Emulsion. Foods. 2023; 12(22):4183. https://doi.org/10.3390/foods12224183
Chicago/Turabian StyleZhang, Yajing, Yu Chen, Chen Liu, Fusheng Chen, and Lijun Yin. 2023. "Effects of Roasting Temperatures on Peanut Oil and Protein Yield Extracted via Aqueous Enzymatic Extraction and Stability of the Oil Body Emulsion" Foods 12, no. 22: 4183. https://doi.org/10.3390/foods12224183