Antioxidant, Antihypertensive, Hypoglycaemic and Nootropic Activity of a Polyphenolic Extract from the Halophyte Ice Plant (Mesembryanthemum crystallinum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Processing
2.3. Extract Preparation
2.4. Determination of Total Phenol Content
2.5. Determination of Total Antioxidant Capacity (TAC)
2.5.1. DPPH Radical Scavenging Activity
2.5.2. Oxygen Radical Absorbance Capacity (ORAC)
2.5.3. Trolox Equivalent Antioxidant Capacity (TEAC)
2.5.4. Ferric Reducing Antioxidant Power (FRAP)
2.5.5. Ferrous Ion Chelating Activity
2.6. Determination of ACE Inhibitory Activity
2.7. Determination of PEP Inhibitory Activity
2.8. Determination of DPP-IV Inhibitory Activity
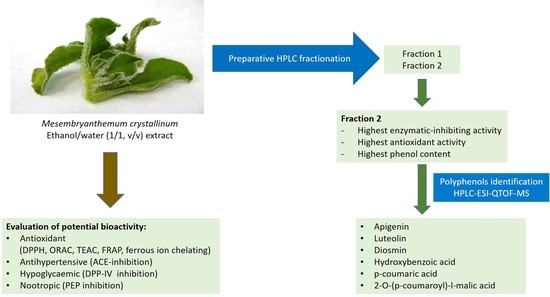
2.9. Chromatographic Fractionation of the Extracts
2.10. Tentative Identification of Polyphenols by HPLC-QTOF
2.11. Statistical Analysis
3. Results and Discussion
3.1. Bioactivity of Extracts
3.1.1. Antioxidant Activity
3.1.2. ACE, PEP and DPP-IV Inhibitory Activity
3.2. Fractionation of the Ice Plant Extract
3.2.1. Antioxidant Activity of the Fractions
3.2.2. ACE, PEP and DPP-IV Inhibitory Activity
3.3. Identification of Polyphenols in the Active Fraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, H. Chronic disease prevention and health promotion. In Public Health Ethics: Cases Spanning the Globe; Barrett, D.H., Ortmann, L.W., Dawson, A., Saenz, C., Reis, A., Bolan, G., Eds.; Springer: New York, NY, USA, 2016; pp. 137–176. Available online: https://link.springer.com/book/10.1007/978-3-319-23847-0 (accessed on 22 May 2022).
- Lee, J.-W.; Park, S.-Y.; Oh, M.-M. Supplemental radiation of ultraviolet-A light-emitting diode improves growth, antioxidant phenolics, and sugar alcohols of ice plant. Hortic. Environ. Biotechnol. 2021, 62, 559–570. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.-K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef] [Green Version]
- Svarcbahs, R.; Julku, U.; Kilpeläinen, T.; Kyyrö, M.; Jäntti, M.; Myöhänen, T.T. New tricks of prolyl oligopeptidase inhibitors—A common drug therapy for several neurodegenerative diseases. Biochem. Pharmacol. 2019, 161, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Babkova, K.; Korabecny, J.; Soukup, O.; Nepovimova, E.; Jun, D.; Kuca, K. Prolyl oligopeptidase and its role in the organism: Attention to the most promising and clinically relevant inhibitors. Future Med. Chem. 2017, 9, 1015–1038. [Google Scholar] [CrossRef]
- Gass, J.; Khosla, C. Biomedicine & Diseases: Review. Prolyl endopeptidases. Cell. Mol. Life Sci. 2007, 64, 345–355. [Google Scholar] [CrossRef]
- Marques, M.R.; Stuker, C.; Kichik, N.; Tarrago, T.; Giralt, E.; Morel, A.F.; Dalcol, I.I. Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers. Fitoterapia 2010, 81, 552–556. [Google Scholar] [CrossRef]
- Kazakos, K. Incretin effect: GLP-1, GIP, DPP4. Diabetes Res. Clin. Pract. 2011, 93, S32–S36. [Google Scholar] [CrossRef]
- Singh, A.-K.; Yadav, D.; Sharma, N.; Jin, J.-O. Dipeptidyl Peptidase (DPP)-IV inhibitors with antioxidant potential isolated from natural sources: A novel approach for the management of diabetes. Pharmaceuticals 2021, 14, 586. [Google Scholar] [CrossRef]
- Atzori, G.; de Vos, A.C.; van Rijsselberghe, M.; Vignolini, P.; Rozema, J.; Mancuso, S.; van Bodegom, P.M. Effects of increased seawater salinity irrigation on growth and quality of the edible halophyte Mesembryanthemum crystallinum L. under field conditions. Agric. Water Manag. 2017, 187, 37–46. [Google Scholar] [CrossRef]
- Loconsole, D.; Murillo-Amador, B.; Cristiano, G.; De Lucia, B. Halophyte common ice plants: A future solution to arable land salinization. Sustainability 2019, 11, 6076. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Gawad, A.M.; Shehata, H.S. Ecology and development of Mesembryanthemum crystallinum L. in the Deltaic Mediterranean coast of Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Lee, C.C.; Wu, S.C. Ice plant (Mesembryanthemum crystallinum) improves hyperglycaemia and memory impairments in a Wistar rat model of streptozotocin-induced diabetes. J. Sci. Food Agric. 2014, 94, 2266–2273. [Google Scholar] [CrossRef] [PubMed]
- Ibtissem, B.; Abdelly, C.; Sfar, S. Antioxidant and antibacterial properties of Mesembryanthemum crystallinum and Carpobrotus edulis extracts. Adv. Chem. Eng. Sci. 2012, 2, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; Gómez-Guillén, M.C.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Sentandreu, M.A.; Toldrá, F.A. Rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Haddar, A.; Gómez-Guillén, M.C.; Nasri, M.; Montero, M.P.; Bougatef, A. Recovery, viscoelastic and functional properties of Barbel skin gelatine: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatine polypeptide. Food Chem. 2015, 168, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Lucas-Abellán, C.; Mercader-Ros, M.T.; Zafrilla, M.P.; Gabaldón, J.A.; Núñez-Delicado, E. Comparative study of different methods to measure antioxidant activity of resveratrol in the presence of cyclodextrins. Food Chem. Toxicol. 2011, 49, 1255–1260. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Ngxabi, S.; Jimoh, M.O.; Kambizi, L.; Laubscher, C.P. Growth characteristics, phytochemical contents, and antioxidant capacity of Trachyandra ciliata (L.f) Kunth grown in hydroponics under varying degrees of salinity. Horticulturae 2021, 7, 244. [Google Scholar] [CrossRef]
- Souid, A.; Croce, C.M.D.; Frassinetti, S.; Gabriele, M.; Pozzo, L.; Ciardi, M.; Abdelly, C.; Hamed, K.B.; Magné, C.; Longo, V. Nutraceutical potential of leaf hydro-ethanolic extract of the edible halophyte Crithmum maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef]
- Boestfleisch, C.; Papenbrock, J. Changes in secondary metabolites in the halophytic putative crop species Crithmum maritimum L., Triglochin maritima L. and Halimione portulacoides (L.) Aellen as reaction to mild salinity. PLoS ONE 2017, 12, e0176303. [Google Scholar] [CrossRef] [Green Version]
- Cybulska, I.; Zembrzuska, J.; Brudecki, G.; Thomsen, M.H. Optimizing methods to characterize caffeic, ferulic, and chlorogenic acids in Salicornia sinus-persica and Salicornia bigelovii extracts by tandem mass spectrometry (LC-MS/MS). Bioresources 2021, 16, 5508–5523. [Google Scholar] [CrossRef]
- Hanen, F.; Riadh, K.; Samia, O.; Sylvain, G.; Christian, M.; Chedly, A. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem. Toxicol. 2009, 47, 2308–2313. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, Y.K.; Lin, Y.H. Photoprotective effects of ice plant (Mesembryanthemum crystallinum) callus extract on gene expression of human dermal fibroblast against UV exposure. J. Biobased Mater. Bioenergy 2019, 13, 570–575. [Google Scholar] [CrossRef]
- Seo, J.A.; Ju, J. Antioxidant and growth inhibitory activities of Mesembryanthemum crystallinum L. in HCT116 human colon cancer cells. J. Nutr. Health 2019, 52, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Sinan, K.I.; Cziaky, Z.; Jeko, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.R.; Mahomoodally, M.F. Assessment of the pharmacological properties and phytochemical profile of Bruguiera gymnorhiza (L.) lam using in vitro studies, in silico docking, and multivariate analysis. Biomolecules 2020, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Shukor, N.A.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef] [PubMed]
- Men, R.; Li, N.; Xing, Y.; Tang, Y.; Tan, C.; Meng, F.; Zhang, J.; Ni, H.; Ji, X. Chemical constituents and ACE inhibitory activity of desert plant Suaeda physophora Pall. Acta Pharm. Sin. B 2013, 3, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y.; Park, K.H.; Hwang, D.Y.; Chanmuang, S.; Jaiswal, L.; Park, Y.K.; Park, S.Y.; Kim, S.Y.; Kim, H.R.; Moon, J.H.; et al. Antihypertensive effects of Artemisia scoparia waldst in spontaneously hypertensive rats and identification of angiotensin I converting enzyme inhibitors. Molecules 2015, 20, 19789–19804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Cacador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of drying processes on the nutritional composition, volatile profile, phytochemical content and bioactivity of Salicornia ramosissima J. woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.M.; Darabi, R.; Akbarloo, N. Study of antihypertensive mechanism of Tribulus terrestris in 2K1C hypertensive rats: Role of tissue ACE activity. Life Sci. 2003, 73, 2963–2971. [Google Scholar] [CrossRef]
- Phillips, O.A.; Mathew, K.T.; Oriowo, M.A. Antihypertensive and vasodilator effects of methanolic and aqueous extracts of Tribulus terrestris in rats. J. Ethnopharmacol. 2006, 104, 351–355. [Google Scholar] [CrossRef]
- Gattringer, J.; Ndogo, O.E.; Retzl, B.; Ebermann, C.; Gruber, C.W.; Hellinger, R. Cyclotides isolated from Violet plants of Cameroon are inhibitors of human Prolyl Oligopeptidase. Front. Pharmacol. 2021, 12, 707596. [Google Scholar] [CrossRef]
- Tarrago, T.; Kichik, N.; Segui, J.; Giralt, E. The natural product berberine is a human prolyl oligopeptidase inhibitor. Chem. Med. Chem. 2007, 2, 354–359. [Google Scholar] [CrossRef]
- Benwahhoud, M.; Jouad, H.; Eddouks, M.; Lyoussi, B. Hypoglycemic effect of Suaeda fruticosa in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2001, 76, 35–38. [Google Scholar] [CrossRef]
- Farias, D.D.; de Araujo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.H.R.; Ahmad, S. Herbs that heal: Natural remedies for health promotion and longevity. Ann. Phytomed. Int. J. 2019, 8, 7–18. [Google Scholar] [CrossRef]
- Calvo, M.M.; Tzamourani, A.; Martínez-Alvarez, O. Halophytes as a potential source of melanosis-inhibiting compounds. Mechanism of inhibition of a characterized polyphenol extract of purslane (Portulaca oleracea). Food Chem. 2021, 355, 129649. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric analysis of phenolic compounds in grapes and wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.B.; Quiles, J.L.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Huang, G. Protein hydrolysates as promoters of non-haem iron absorption. Nutrients 2017, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115. [Google Scholar]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Pharmacology of diosmin, a citrus flavone glycoside: An updated review. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current state of knowledge on the antioxidant effects and mechanisms of action of polyphenolic compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Błaszczaka, W.; Latochab, P.; Jeża, M.; Wiczkowskia, W. The impact of high-pressure processing on the polyphenol profile and anti-glycaemic, anti-hypertensive and anti-cholinergic activities of extracts obtained from kiwiberry (Actinidia arguta) fruits. Food Chem. 2021, 343, 128421. [Google Scholar] [CrossRef]
- Shree, V.S.; Sathishkumar, T.; Kumaresan, K.; Rapheal, V.S.; Muthukumaran, P.; Muthukumaran, V. Therapeutic effects of purified polyphenols from Coccinia grandis: Correlation between hypertension and diabetes mellitus. Adv. Tradit. Med. 2021, 21, 579–590. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quinones, M.; Garcia-Vallve, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Ullah, K.; Siddiqui, H.; Iqbal, S.; Wahab, A.; Goren, N.; Ayatollahi, S.A.; Rahman, A.; Choudhary, M.I. Chemical constituents from Parrotia persica- Structural derivatization and their potential prolyl endopeptidase inhibition activity. Bioorg. Chem. 2020, 96, 103526. [Google Scholar] [CrossRef]
- Cui, T.; Nakamura, K.; Tian, S.; Kayahara, H.; Tian, Y.L. Polyphenolic content and physiological activities of Chinese hawthorn extracts. Biosci. Biotechnol. Biochem. 2006, 70, 2948–2956. [Google Scholar] [CrossRef] [Green Version]
- Tarragó, T.; Kichik, N.; Claasen, B.; Prades, R.; Teixidó, M.; Giralt, E. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg. Med. Chem. 2008, 16, 7516–7524. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, S.-I.; Song, K.-S. Prolyl Endopeptidase inhibitors from green tea. Arch. Pharm. Res. 2001, 24, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.Z.; Tezuka, Y.; Komatsu, K.; Namba, T.; Kadota, S. Prolyl endopeptidase inhibitors from the underground part of Rhodiola sacra S.H. Fu. Biol. Pharm. Bull. 1999, 22, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Jun, M.; Choi, J.-Y.; Yang, E.-J.; Hur, J.-M.; Bae, K.; Seong, Y.-H.; Huh, T.-L.; Song, K.-S. Plant phenolics as Prolyl Endopeptidase inhibitors. Arch. Pharm. Res. 2007, 30, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Kwak, J.H.; Lee, K.-B.; Song, K.-S. Prolyl Endopeptidase inhibitors from Caryophylli Flos. Arch. Pharm. Res. 1998, 21, 207–211. [Google Scholar] [CrossRef]
- Gao, F.; Fu, Y.; Yi, J.; Gao, A.; Jia, Y.; Cai, S. Effects of different dietary flavonoids on Dipeptidyl Peptidase-IV activity and expression: Insights into structure-activity relationship. J. Agric. Food Chem. 2020, 68, 12141–12151. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; Gonzalez de Mejia, E. Berry and citrus phenolic compounds inhibit Dipeptidyl Peptidase IV: Implications in diabetes management. Evid. Based Complement. Altern. Med. 2013, 479505. [Google Scholar] [CrossRef] [Green Version]
- Bower, A.M.; Real Hernandez, L.M.; Berhow, M.A.; Gonzalez de Mejia, E. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef]
- Haron, N.; Nadia, N.; Farahin, P.N.; Susanti, D.; Hasniza, N.; Bariyya, K.; Halim, A. Molecular docking of polyphenol compounds from Anacardium occidentale with alpha-glucosidase and dipeptidyl-peptidase-4 enzymes. Malays. J. Fundam. Appl. Sci. 2021, 17, 202–216. [Google Scholar]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive compounds effective against type 2 Diabetes Mellitus: A systematic review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef]



| Assay | M. crystallinum Extract |
|---|---|
| Chemical composition | |
| Total phenolic compounds (mEq GA/g) | 10.02 ± 0.07 |
| Antioxidant activity | |
| DPPH (µEq Trolox/g) | 21.0 ± 0.3 |
| ORAC (µEq Trolox/g) | 88.0 ± 10.4 |
| TEAC (µEq Trolox/g) | 84.4 ± 4.6 |
| FRAP (mEq Mohr’s salt/g) | 8.9 ± 0.3 |
| Ferrous ion chelating activity (mEq EDTA/g) | 1.6 ± 0.0 |
| Enzymatic activity inhibition | |
| ACE, 1 mg/mL (%) | 90.5 ± 3.3 |
| PEP, 1 mg/mL (%) | 98.6 ± 0.1 |
| DPP-IV, 1 mg/mL (%) | 73.1 ± 3.3 |
| Assay | Fraction 1 | Fraction 2 |
|---|---|---|
| Chemical composition | ||
| Total phenols (mE(q GAE/g) | 9.28 ± 2.8 a | 61.9 ± 2.8 b |
| Antioxidant activity | ||
| DPPH (μEq Trolox/g) | 64.4 ± 9.3 a | 185.3 ± 53.6 b |
| ORAC (μEq Trolox/g) | 427.9 ± 42.0 a | 1541.5 ± 78.0 b |
| TEAC (μEq Trolox/g) | 739.2 ± 88.1 a | 1341.7 ± 75.3 b |
| FRAP (mEq Mohr’s salt/g) | 6.4 ± 0.0 a | 13.5 ± 0.1 b |
| Ferrous ion chelating activity (mEq EDTA/g) | 4.8 ± 0.1 a | 4.4 ± 0.1 b |
| Enzymatic activity inhibition | ||
| ACE, 100 µg/mL (%) | 59.3 ± 1.69 a | 100 ± 0.0 b |
| PEP, 200 µg/mL (%) | 0 a | 90.6 ± 2.5 b |
| DPP-IV, 200 µg/mL (%) | 11.7 ± 1.5 a | 58.7 ± 0.7 b |
| Rt (Min) | Proposed Compound | Experimental Mass | Calculated Mass | Error (ppm) | Ms/Ms Ions | Relative Area (%) |
|---|---|---|---|---|---|---|
| Hidroxybenzoic acids | ||||||
| 10.9 | 4-Hydroxybenzoic acid | 137.0238 | 137.0241 | −2.41 | 65, 69, 93, 109,119, 137 | 7.49 |
| Hydroxycinnamic Acids | ||||||
| 17.3 | p-Coumaric acid | 163.0401 | 163.0404 | −3.64 | 65, 67, 75, 88, 119, 137, 145, 163 | 12.00 |
| Hydroxycinnamic Acids derivatives | ||||||
| 17.8 | 2-O-(p-Coumaroyl)-l-malic acid | 279.051 | 279.0521 | −3.38 | 71, 89, 115, 119, 133, 145,1 63, 189 | 18.60 |
| Flavonoids | ||||||
| 22.0 | Diosmin | 607.1668 | 607.1674 | −1.60 | 284, 285, 299, 300, 301 | 17.72 |
| 28.7 | Luteolin | 285.0405 | 285.0409 | −1.38 | 83, 107, 133, 143, 149, 151, 175, 199, 217 | 11.90 |
| 33.5 | Apigenin | 269.0455 | 269.0463 | −2.68 | 107, 118, 121, 149, 151, 158, 225, 269 | 38.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, M.M.; Martín-Diana, A.B.; Rico, D.; López-Caballero, M.E.; Martínez-Álvarez, O. Antioxidant, Antihypertensive, Hypoglycaemic and Nootropic Activity of a Polyphenolic Extract from the Halophyte Ice Plant (Mesembryanthemum crystallinum). Foods 2022, 11, 1581. https://doi.org/10.3390/foods11111581
Calvo MM, Martín-Diana AB, Rico D, López-Caballero ME, Martínez-Álvarez O. Antioxidant, Antihypertensive, Hypoglycaemic and Nootropic Activity of a Polyphenolic Extract from the Halophyte Ice Plant (Mesembryanthemum crystallinum). Foods. 2022; 11(11):1581. https://doi.org/10.3390/foods11111581
Chicago/Turabian StyleCalvo, Marta María, Ana Belén Martín-Diana, Daniel Rico, María Elvira López-Caballero, and Oscar Martínez-Álvarez. 2022. "Antioxidant, Antihypertensive, Hypoglycaemic and Nootropic Activity of a Polyphenolic Extract from the Halophyte Ice Plant (Mesembryanthemum crystallinum)" Foods 11, no. 11: 1581. https://doi.org/10.3390/foods11111581