Effects of Long-Term Bottle Storage on Red and Rosé Wines Sealed with Different Types of Closures
Abstract
:1. Introduction
2. Materials and Methods
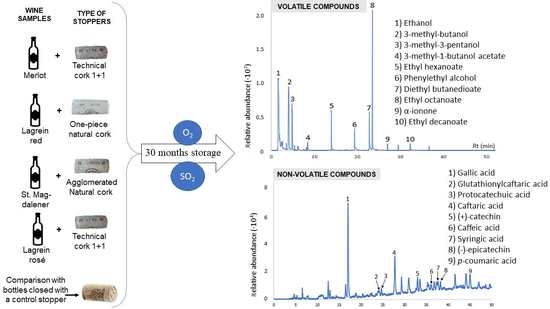
2.1. Wines and Closures
2.2. Analysis of Volatile Compounds
2.3. HPLC Analysis
2.4. Determination of Dissolved O2 and SO2
2.5. Sensory Discrimination Test
2.6. Multivariate Data Analysis
3. Results
3.1. Profile of Volatile Compounds
3.2. Non-Volatile Compounds
3.3. Sulfur Dioxide and Dissolved Oxygen Content
3.4. Correlations between Sulfur Dioxide, Dissolved Oxygen and Volatile and Phenolic Profiles
3.5. Sensory Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A. Handbook of Enology, Volume 2: The Chemistry of Wine-Stabilization and Treatments (Volume 2); Dubourdieu, D., Ed.; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Hernanz, D.; Gallo, V.; Ángeles, F.R.; Meléndez-Martínez, A.J.; González-Miret, M.L.; Heredia, F.J. Effect of storage on the phenolic content, volatile composition and colour of white wines from the varieties Zalema and Colombard. Food Chem. 2009, 113, 530–537. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Varea, S.; García-Vallejo, M.; Cadahia, E.; de Simón, F.B. Polyphenols susceptible to migrate from cork stoppers to wine. Eur. Food Res. Technol. 2001, 213, 56–61. [Google Scholar] [CrossRef]
- Skouroumounis, G.; Kwiatkowski, M.; Francis, I.; Oakey, H.; Capone, D.; Duncan, B.; Sefton, M.; Waters, E. The impact of closure type and storage conditions on the composition, colour and flavour properties of a Riesling and a wooded Chardonnay wine during five years’ storage. Aust. J. Grape Wine Res. 2005, 11, 369–377. [Google Scholar] [CrossRef]
- González-Adrados, J.R.; González-Hernández, F.; De Ceca, J.L.G.; Cáceres-Esteban, M.J.; García-Vallejo, M.C. Cork-wine interaction studies: Liquid absorption and non-volatile compound migration. OENO One 2008, 42, 163–166. [Google Scholar] [CrossRef]
- Azevedo, J.; Fernandes, I.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; Freitas, V. Migration of phenolic compounds from different cork stoppers to wine model solutions: Antioxidant and biological relevance. Eur. Food Res. Technol. 2014, 239, 951–960. [Google Scholar] [CrossRef]
- Waters, E.; Peng, Z.; Pocock, K.; Williams, P. The role of corks in oxidative spoilage of white wines. Aust. J. Grape Wine Res. 1996, 2, 191–197. [Google Scholar] [CrossRef]
- Mas, A.; Puig, J.; Lladoa, N.; Zamora, F. Sealing and Storage Position Effects on Wine Evolution. J. Food Sci. 2002, 67, 1374–1378. [Google Scholar] [CrossRef]
- Lopes, P.; Saucier, C.; Teissedre, A.P.-L.; Glories, Y. Impact of Storage Position on Oxygen Ingress through Different Closures into Wine Bottles. J. Agric. Food Chem. 2006, 54, 6741–6746. [Google Scholar] [CrossRef]
- Karbowiak, T.; Gougeon, R.; Alinc, J.-B.; Brachais, L.; Debeaufort, F.; Voilley, A.; Chassagne, D. Wine Oxidation and the Role of Cork. Crit. Rev. Food Sci. Nutr. 2009, 50, 20–52. [Google Scholar] [CrossRef]
- Silva, M.A.; Julien, M.; Jourdes, M.; Teissedre, P.-L. Impact of closures on wine post-bottling development: A review. Eur. Food Res. Technol. 2011, 233, 905–914. [Google Scholar] [CrossRef]
- Ugliano, M. Oxygen Contribution to Wine Aroma Evolution during Bottle Aging. J. Agric. Food Chem. 2013, 61, 6125–6136. [Google Scholar] [CrossRef] [PubMed]
- Gambuti, A.; Siani, T.; Picariello, L.; Rinaldi, A.; Lisanti, M.T.; Ugliano, M.; Dieval, J.B.; Moio, L. Oxygen exposure of tannins-rich red wines during bottle aging. Influence on phenolics and color, astringency markers and sensory attributes. Eur. Food Res. Technol. 2017, 243, 669–680. [Google Scholar] [CrossRef]
- Marais, J.; Pool, H.J. Effect of storage time and temperature on the volatile composition and quality of dry white table wines. Vitis 1980, 19, 151–164. [Google Scholar]
- Simpson, R.F.; Miller, G.C. Aroma composition of aged Riesling wine. Vitis 1983, 22, 2251–2263. [Google Scholar]
- Ugliano, M.; Kwiatkowski, M.J.; Travis, B.; Francis, I.L.; Waters, E.J.; Herderich, M.J.; Pretorius, I.S. Post-bottling management of oxygen to reduce off-flavour formation and optimize wine style. Aust. N. Zealand Wine Ind. J. 2009, 24, 24–28. [Google Scholar]
- Hart, A.; Kleinig, A. The role of oxygen in the aging of bottled wine. Aust. N. Zealand Wine Industry J. 2005, 20, 46–50. [Google Scholar]
- Lopes, P.; Silva, M.A.; Pons, A.; Tominaga, T.; Lavigne, V.; Saucier, C.; Dubourdieu, D. Impact of oxygen dis-solved at bottling and transmitted through closures on the composition and sensory properties of a Sauvignon blanc wine during bottle storage. J. Agric. Food Chem. 2009, 57, 10261–10270. [Google Scholar] [CrossRef]
- Escudero, A.; Asensio, E.; Cacho, J.; Ferreira, V. Sensory and chemical changes of young white wines stored under oxygen. An assessment of the role played by aldehydes and some other important odorants. Food Chem. 2002, 77, 325–331. [Google Scholar] [CrossRef]
- Mozzon, M.; Savini, S.; Boselli, E.; Thorngate, J.H. The herbaceous character of wines. Ital. J. Food Sci. 2016, 28, 190. [Google Scholar]
- Skouroumounis, G.; Kwiatkowski, M.; Francis, I.; Oakey, H.; Capone, D.; Peng, Z.; Duncan, B.; Sefton, M.; Waters, E. The influence of ascorbic acid on the composition, colour and flavour properties of a Riesling and a wooded Chardonnay wine during five years’ storage. Aust. J. Grape Wine Res. 2005, 11, 355–368. [Google Scholar] [CrossRef]
- Arapitsas, P.; Dalledonne, S.; Scholz, M.; Catapano, A.; Carlin, S.; Mattivi, F. White wine light-strike fault: A comparison between flint and green glass bottles under the typical supermarket conditions. Food Packag. Shelf Life 2020, 24, 100492. [Google Scholar] [CrossRef]
- Fracassetti, D.; Gabrielli, M.; Encinas, J.; Manara, M.; Pellegrino, L.M.; Tirelli, A. Approaches to prevent the light-struck taste in white wine. Aust. J. Grape Wine Res. 2017, 23, 329–333. [Google Scholar] [CrossRef]
- Benítez, P.; Castro, R.; Natera, R.; Barroso, C.G. Changes in the polyphenolic and volatile content of “Fino” Sherry wine exposed to high temperature and ultraviolet and visible radiation. Eur. Food Res. Technol. 2006, 222, 302–309. [Google Scholar] [CrossRef]
- Betnga, P.F.T.; Longo, E.; Poggesi, S.; Boselli, E. Effects of transport conditions on the stability and sensory quality of wines. OENO One 2021, 55, 4524. [Google Scholar] [CrossRef]
- Chung, H.-J.; Son, J.-H.; Park, E.-Y.; Kim, E.-J.; Lim, S.-T. Effect of vibration and storage on some physico-chemical properties of a commercial red wine. J. Food Compos. Anal. 2008, 21, 655–659. [Google Scholar] [CrossRef]
- Chatonnet, P.; Labadie, D.; Gubbiotti, M.-C. Comparative study of different types of corkage. Rev. Oeno-Logues Tech. Vitivinic. Oenologiques 2000, 95, 7–13. [Google Scholar]
- Godden, P.; Francis, L.; Field, J.; Gishen, M.; Coulter, A.; Valente, P.; Høj, P.; Robinson, E. Wine bottle closures: Physical characteristics and effect on composition and sensory properties of a Semillon wine 1. Performance up to 20 months post-bottling. Aust. J. Grape Wine Res. 2001, 7, 64–105. [Google Scholar] [CrossRef]
- Godden, P.; Lattey, K.; Francis, L.; Gishen, M.; Cowey, G.; Holdstock, M.; Capone, D. Towards offering wine to the consumer in optimal condition—The wine, the closures and other packaging variables. Wine Ind. J. 2005, 20, 20–30. [Google Scholar]
- Kwiatkowski, M.J.; Skouroumounis, G.K.; Lattey, K.A.; Waters, E.J. The impact of closures, including screw cap with three different headspace volumes, on the composition, colour and sensory properties of a Cabernet Sauvignon wine during two years’ storage. Aust. J. Grape Wine Res. 2007, 13, 81–94. [Google Scholar] [CrossRef]
- Liu, N.; Song, Y.-Y.; Dang, G.-F.; Ye, D.-Q.; Gong, X.; Liu, Y.-L. Effect of Wine Closures on the Aroma Properties of Chardonnay Wines after Four Years of Storage. South Afr. J. Enol. Vitic. 2015, 36, 296–303. Available online: http://www.scielo.org.za/pdf/sajev/v36n3/07.pdf (accessed on 24 November 2021). [CrossRef] [Green Version]
- Rossetti, F.; Jouin, A.; Jourdes, M.; Teissedre, P.-L.; Foligni, R.; Longo, E.; Boselli, E. Impact of Different Stoppers on the Composition of Red and Rosé Lagrein, Schiava (Vernatsch) and Merlot Wines Stored in Bottle. Molecules 2020, 25, 4276. [Google Scholar] [CrossRef] [PubMed]
- Longo, E.; Rossetti, F.; Jouin, A.; Teissedre, P.-L.; Jourdes, M.; Boselli, E. Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines. Food Chem. 2019, 299, 125125. [Google Scholar] [CrossRef] [PubMed]
- De Matos, A.D.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Sanoll, C.; Robatscher, P.; Boselli, E. Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles. Foods 2020, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Klesk, K.; Qian, M. Aroma Extract Dilution Analysis of Cv. Marion (Rubus spp. hyb) and Cv. Evergreen (R. laciniatus L.) Blackberries. J. Agric. Food Chem. 2003, 51, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sun, B.; Zheng, F.; Wang, S. Volatile flavor constituents in roasted pork of Mini-pig. Food Chem. 2008, 109, 506–514. [Google Scholar] [CrossRef]
- Krist, S.; Stuebiger, G.; Unterweger, H.; Bandion, F.; Buchbauer, G. Analysis of Volatile Compounds and Triglycerides of Seed Oils Extracted from Different Poppy Varieties (Papaver somniferum L.). J. Agric. Food Chem. 2005, 53, 8310–8316. [Google Scholar] [CrossRef]
- Iraqi, R.; Vermeulen, C.; Benzekri, A.; Bouseta, A.; Collin, S. Screening for Key Odorants in Moroccan Green Olives by Gas Chromatography−Olfactometry/Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2005, 53, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Shin, J.H.; Yu, E.J.; Kim, Y.-S.; Lee, H.J.; Kim, T.H. Characteristics of aroma-active compounds in the pectin-elicited suspension culture of Zanthoxylum piperitum (prickly ash). Biotechnol. Lett. 2002, 24, 551–556. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grimm, C.C. Identification of Volatile Compounds in Cantaloupe at Various Developmental Stages Using Solid Phase Microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Sampaio, T.S.; Nogueira, P.C.L. Volatile components of mangaba fruit (Hancornia speciosa Gomes) at three stages of maturity. Food Chem. 2006, 95, 606–610. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook, SRD 69. Available online: https://webbook.nist.gov/cgi/inchi/InChI%3D1S/C8H16O3/c1-6(2)4-5-11-8(10)7(3)9/h6-7%2C9H%2C4-5H2%2C1-3H3 (accessed on 24 November 2021).
- Xu, X.; Stee, L.L.P.; Williams, J.; Beens, J.; Adahchour, M.; Vreuls, R.J.J.; Brinkman, U.A.; Lelieveld, J. Comprehensive two-dimensional gas chromatography (GC × GC) measurements of volatile organic compounds in the atmosphere. Atmos. Chem. Phys. Discuss. 2003, 3, 665–682. [Google Scholar] [CrossRef] [Green Version]
- Schirack, A.V.; Drake, M.A.; Sanders, T.H.; Sandeep, K.P. Characterization of aroma-active compounds in microwave blanched peanuts. J. Food Sci. 2006, 71, C513–C520. [Google Scholar] [CrossRef]
- Passos, X.S.; Castro, A.C.M.; Pires, J.S.; Garcia, A.C.F.; Campos, F.C.; Fernandes, O.F.L.; de Paula, J.R.; Ferreira, H.D.; Santos, S.C.; Ferri, P.H.; et al. Composition and Antifungal Activity of the Essential Oils of Caryocar brasiliensis. Pharm. Biol. 2003, 41, 319–324. [Google Scholar] [CrossRef]
- Su, Y.C.; Ho, C.L.; Wang, E.I.; Chang, S.T. Antifungal activities and chemical compositions of essential oils from leaves of four eucalypts. Taiwan J. For. Sci. 2006, 21, 49–61. [Google Scholar]
- Flamini, G.; Cioni, P.L.; Morelli, I. Volatiles from Leaves, Fruits, and Virgin Oil from Olea europaea Cv. Olivastra Seggianese from Italy. J. Agric. Food Chem. 2003, 51, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Lu, W.; Yao, X.; Zhang, Y.; Wang, K.; Pan, S. Effect of fermentation on free and bound volatile compounds of orange juice. Flavour Fragr. J. 2009, 24, 219–225. [Google Scholar] [CrossRef]
- Boulanger, R.; Crouzet, J. Free and bound flavour components of Amazonian fruits: 3-glycosidically bound components of cupuacu. Food Chem. 2000, 70, 463–470. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Betnga, P.F.T.; de Matos, A.D.; Longo, E.; Boselli, E. Impact of closure material on the chemical and sensory profiles of grappa during storage in bottle. LWT 2020, 133, 110014. [Google Scholar] [CrossRef]
- Bowen, A.J.; Reynolds, A.G. Odor Potency of Aroma Compounds in Riesling and Vidal blanc Table Wines and Icewines by Gas Chromatography–Olfactometry–Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 2874–2883. [Google Scholar] [CrossRef]
- Xing, R.R.; Liu, D.; Li, Z.; Tian, Y.; Zhang, X.X.; Li, J.M.; Pan, Q.H. Impact of different types of closures on sensorial and phenolic characteristics evolution during a bottle storage time of a white wine from Chardonnay grape variety. J. Food Sci. Technol. 2016, 53, 4043–4055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Lambri, M.; De Faveri, M.D. Evaluation of the performances of synthetic and cork stoppers up to 24 months post-bottling. Eur. Food Res. Technol. 2003, 216, 529–534. [Google Scholar] [CrossRef]

| Wines | Type of Closures | Images of Closures |
|---|---|---|
| Merlot | Blend cork or technical cork (1 + 1) |  or |
| Lagrein red | Blend cork or natural one-piece cork |  or |
| Lagrein rosé | Blend cork or technical cork (1 + 1) |  or |
| St. Magdalener | Blend cork or agglomerated natural cork (0 + 0) |  or |
| Volatile Compounds | Over 12 Months Storage as in [33] | At a 30-Month Storage | LRI (Ref./NIST) | LRI (Measured) | Base Peak (m/z) | Fragmentation Pattern (m/z) | |
|---|---|---|---|---|---|---|---|
| 1 | Acetic acid | √ | √ | 599 [36] | / | 43 | 43; 45; 60 |
| 2 | 3-methyl-1-butanol | X | √ | 732 [37] | 705 | 55 | 42; 55; 70 |
| 3 | 2,3-butanediol | X | √ | 782 [38] | 782 | 45 | 45; 57 |
| 4 | Ethyl butanoate | √ | √ | 803 [36] | 772 | 71 | 43; 71; 88 |
| 5 | 2-hydroxyethyl propanoate | X | √ | 793 [39] | 792 | 57 | 45; 57; 75; 87; 88 |
| 6 | Ethyl ester of 2-methylbutanoic acid | √ | √ | 846 [36] | 824 | 57 | 57; 74; 85; 102 |
| 7 | Ethyl ester of 3-methylbutanoic acid | √ | √ | 859 [40] | 827 | 88 | 41; 57; 70; 88 |
| 8 | 1-Hexanol | √ | √ | 865 [41] | 843 | 56 | 56; 69; 84 |
| 9 | Isopentyl acetate | √ | √ | 876 [41] | 850 | 43 | 43; 55; 70 |
| 10 | 2-butylester of 4-ethylbenzoic acid | √ | X | / | / | 133 | 105; 151 |
| 11 | 1-Heptanol | √ | X | 969 [41] | / | 70 | 41; 42; 43; 55; 56; 70 |
| 12 | 1-Octen-3-ol | √ | X | 980 [36] | / | 57 | 43; 55; 57; 72 |
| 13 | Ethyl hexanoate | √ | √ | 999 [41] | 975 | 88 | 43; 60; 70; 88; 99 |
| 14 | Hexyl acetate | √ | X | 1011 [41] | / | 43 | 43; 55; 56; 61; 69; 84 |
| 15 | Limonene | √ | X | 1020 [41] | / | 68 | 67; 68; 93 |
| 16 | 2-Ethyl hexanol | √ | X | 1028 [42] | / | 57 | 41; 43; 57 |
| 17 | Isoamyl lactate | X | √ | 1047 [43] | 1042 | 45 | 43; 45; 55; 70 |
| 18 | 4-Methyl benzaldehyde | √ | X | 1076 [44] | / | 91 | 65; 91; 119; 120 |
| 19 | Octanol | √ | √ | 1070 [41] | 1055 | 56 | 41; 42; 43; 55; 56; 69; 70; 84 |
| 20 | 4-Ethylbenzaldehyde | √ | X | 1163 [45] | / | 134 | 91; 105; 133; 134 |
| 21 | 2-Phenylethanol | √ | √ | 1112 [36] | 1084 | 91 | 65; 91; 122 |
| 22 | Octanoic acid | √ | √ | 1180 [46] | 1152 | 60 | 60; 73; 101 |
| 23 | Diethyl succinate | √ | √ | 1179 [47] | 1155 | 101 | 45; 55; 73; 101; 129 |
| 24 | Methyl salicylate | √ | X | 1192 [41] | / | 120 | 92; 120; 121; 152 |
| 25 | Ethyl octanoate | √ | √ | 1194 [48] | 1169 | 88 | 41; 57; 73; 88; 101; 115; 127 |
| 26 | Benzeneacetic acid, ethyl ester | √ | X | 1243 [41] | / | 91 | 164; 91;65 |
| 27 | 2-Phenylethylacetate | √ | X | 1255 [41] | / | 104 | 43; 91; 104 |
| 28 | Ethyl decanoate | √ | √ | 1403 [49] | 1361 | 88 | 73; 88; 101; 155 |
| 29 | Ethyl dodecanoate | √ | X | 1554 [41] | / | 88 | 43; 73; 88; 101 |
| 30 | Ethyl hexadecanoate | √ | X | 1992 [50] | / | 88 | 43; 88; 101 |
| Merlot | ||||||||||
| Closure | 3-Methyl-1-Butanol | 2-Hydroxyethyl Propanoate | 2-Methyl ethyl Butanoate | 3-Methyl ethyl Butanoate | 1-Hexanol | Isopenthyl Acetate | Ethyl Hexanoate | Isoamyl Lactate | 2-Phenyl Ethanol | |
| C | 622245228 | 40645189 | 3806540 | 10057235 | 15511016 | 17334871 | 201290040 | 3860017 | 98766115 | |
| B | 820647919 | 46158129 | 5210750 | 13167523 | 20750259 | 39098612 | 192166261 | 4403087 | 175713260 | |
| Pr > F | 0.341 | 0.865 | 0.643 | 0.746 | 0.315 | 0.242 | 0.804 | 0.491 | 0.501 | |
| closure | Diethyl succinate | Octanoic acid | Ethyl octanoate | Ethyl decanoate | ||||||
| C | 532233181 | 128705315 | 430186222 | 16189337 | ||||||
| B | 249480078 | 4616469 | 510187046 | 31310515 | ||||||
| Pr > F | 0.423 | 0.415 | 0.862 | 0.568 | ||||||
| Lagrein red | ||||||||||
| Closure | 3-Methyl-1-butanol | 2,3-Butanediol | 2-Hydroxyethyl propanoate | 1-Hexanol | Isopentyl acetate | Ethyl hexanoate | 2-Phenylethanol | Diethyl succinate | Ethyl octanoate | Ethyl decanoate |
| C | 608185318 | 12511497 | 24631214 | 23588648 | 73852849 | 241125725 | 163376742 | 279803394 | 987171673 | 98798491 |
| B | 808285867 | 806359 | 37373403 | 22647330 | 60874527 | 189831224 | 129556887 | 237468679 | 535496801 | 42955096 |
| Pr > F | 0.691 | 0.006 * | 0.166 | 0.809 | 0.214 | 0.137 | 0.179 | 0.223 | 0.010 * | 0.253 |
| Lagrein rosé | ||||||||||
| Closure | 3-Methyl-1-butanol | 2,3-Butanediol | 1-Hexanol | Isopentyl acetate | Ethyl hexanoate | 2-Phenylethanol | Diethyl succinate | Ethyl octanoate | Ethyl decanoate | |
| C | 181321516 | 7388017 | 43588910 | 58215889 | 540146675 | 41387280 | 158422635 | 2441116143 | 4952211955 | |
| B | 297558079 | 47935843 | 45742412 | 63857184 | 509214725 | 70638784 | 210194281 | 1614962842 | 221721242 | |
| Pr > F | 0.118 | 0.487 | 0.550 | 0.792 | 0.436 | 0.359 | 0.331 | 0.065 | 0.131 | |
| St. Magdalener | ||||||||||
| Closure | Acetic acid | 3-Methyl-1-butanol | 2,3-Butanediol | Ethyl butanoate | 2-Hydroxyethyl propanoate | 2-Methyl ethyl butanoate | 3-Methyl ethyl butanoate | 1-Hexanol | Isopenthyl acetate | |
| C | 11505643 | 889501476 | 2914012 | 5280037 | 35636365 | 4369199 | 10416494 | 23082888 | 83015919 | |
| B | 0 | 754427998 | 6749973 | 4663781 | 39471604 | 3705953 | 10245563 | 24749296 | 74859273 | |
| Pr > F | 0.414 | 0.533 | 0.342 | 0.751 | 0.574 | 0.382 | 0.835 | 0.234 | 0.701 | |
| Closure | Ethyl hexanoate | Isoamyl lactate | Octanol | 2-Phenyl ethanol | Diethyl succinate | Octanoic acid | Ethyl octanoate | Ethyl decanoate | ||
| C | 224036589 | 5092575 | 5513034 | 227373202 | 354489074 | 5627357 | 659384377 | 22794356 | ||
| B | 189855333 | 30786045 | 16732152 | 158471891 | 252616407 | 4325906 | 507270573 | 24993361 | ||
| Pr > F | 0.151 | 0.425 | 0.419 | 0.223 | 0.129 | 0.789 | 0.143 | 0.825 | ||
| Closure | Gallic Acid | Protocatechuic Acid | Caftaric Acid | Glutathionyl Caftaric Acid | (+)-Catechin | Caffeic Acid | Syringic Acid | (−)-Epicatechin | p-Coumaric Acid | Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|---|
| Merlot | ||||||||||
| C | 797902 | 19277 | 452477 | 64174 | 161170 | 109558 | 85709 | 92869 | 190434 | 86965 |
| B | 775296 | 20349 | 442758 | 68093 | 143295 | 96415 | 91451 | 80817 | 179824 | 50231 |
| Pr > F | 0.065 | 0.063 | 0.308 | 0.001 * | 0.039 * | 0.193 | 0.045 * | 0.003 * | 0.371 | 0.157 |
| Lagrein red | ||||||||||
| C | 529893 | 26416 | 534064 | 56755 | 116984 | 251583 | 179799 | 77357 | 349054 | 184361 |
| B | 547415 | 29802 | 554889 | 61792 | 117628 | 244212 | 212296 | 100352 | 378623 | 133896 |
| Pr > F | 0.234 | 0.026 * | 0.459 | 0.026 * | 0.956 | 0.869 | 0.068 | 0.229 | 0.522 | 0.278 |
| Lagrein rosé | ||||||||||
| C | 38832 | 9592 | 90022 | 24781 | 33893 | 20066 | 31342 | 6048 | 46235 | 16893 |
| B | 38850 | 10169 | 88684 | 25115 | 37145 | 17456 | 32832 | 3878 | 41259 | 11602 |
| Pr > F | 0.966 | 0.637 | 0.463 | 0.668 | 0.078 | 0.051 | 0.102 | 0.040 * | 0.137 | 0.133 |
| St. Magdalener | ||||||||||
| C | 144676 | 17699 | 471285 | 66081 | 173598 | 135204 | 83950 | 82501 | 245319 | 171631 |
| B | 143419 | 19867 | 474859 | 76162 | 106769 | 78370 | 85387 | 60521 | 253125 | 77102 |
| Pr > F | 0.848 | 0.082 | 0.748 | 0.005 * | 0.056 | 0.004 * | 0.807 | 0.001 * | 0.250 | 0.014 * |
| Closures | Total Sulfur Dioxide (mg/L) | Free Sulfur Dioxide (mg/L) | Oxygen Content (mg/L) |
|---|---|---|---|
| Merlot | |||
| C | 38.50 | 26.00 | 1.85 |
| B | 26.50 | 20.00 | 1.05 |
| Pr > F | 0.077 | 0.238 | 0.064 |
| Lagrein red | |||
| C | 37.00 | 22.50 | 0.15 |
| B | 28.50 | 25.00 | 0.10 |
| Pr > F | 0.054 | 0.649 | 0.423 |
| Lagrein rosé | |||
| C | 96.00 | 21.00 | 2.00 |
| B | 83.00 | 12.50 | 2.00 |
| Pr > F | 0.049 | 0.003 * | 1.000 |
| St. Magdalener | |||
| C | 36.00 | 16.00 | 0.25 |
| B | 19.00 | 11.50 | 0.05 |
| Pr > F | 0.033 | 0.057 | 0.106 |
| Merlot | Lagrein Red | Lagrein Rosé | St. Magdalener |
|---|---|---|---|
| ns | ns | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchouakeu Betnga, P.F.; Longo, E.; Merkytė, V.; Dupas de Matos, A.; Rossetti, F.; Boselli, E. Effects of Long-Term Bottle Storage on Red and Rosé Wines Sealed with Different Types of Closures. Foods 2021, 10, 2918. https://doi.org/10.3390/foods10122918
Tchouakeu Betnga PF, Longo E, Merkytė V, Dupas de Matos A, Rossetti F, Boselli E. Effects of Long-Term Bottle Storage on Red and Rosé Wines Sealed with Different Types of Closures. Foods. 2021; 10(12):2918. https://doi.org/10.3390/foods10122918
Chicago/Turabian StyleTchouakeu Betnga, Prudence Fleur, Edoardo Longo, Vakarė Merkytė, Amanda Dupas de Matos, Fabrizio Rossetti, and Emanuele Boselli. 2021. "Effects of Long-Term Bottle Storage on Red and Rosé Wines Sealed with Different Types of Closures" Foods 10, no. 12: 2918. https://doi.org/10.3390/foods10122918