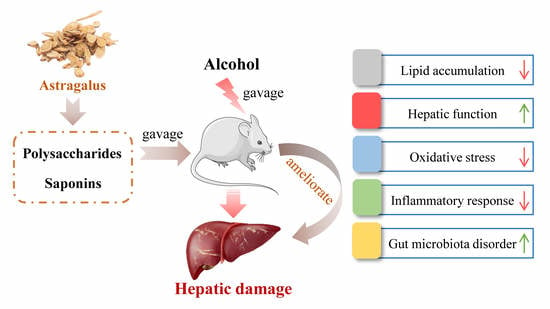
Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Astragalus Saponins and Polysaccharides
2.3. Animals and Experimental Design
2.4. Measurement of Biochemical Indicators in Serum and Liver
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Histological Analysis
2.7. Intestinal Microbiota Analysis
2.8. Statistical Analysis
3. Result
3.1. Effect of AP and AS on Food Intake, Body Weight, and Organ and Fat Index in ALD Mice
3.2. Effect of AP and AS on Lipids of Serum and Liver in ALD Mice
3.3. Effect of AP and AS on the Hepatic Function in ALD Mice
3.4. Effect of AP and AS on the Liver Oxidative Stress in ALD Mice
3.5. Effect of AP and AS on Inflammation Response in ALD Mice
3.6. Effect of AP and AS on Oxidative Stress and Inflammation-Related Gene Expression in ALD Mice
3.7. Effect of AP and AS on Histopathological Variations of Livers in ALD Mice
3.8. Effects of AP and AS on the Changes of the Colonic Microbiota Composition in ALD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Roth, N.C.; Qin, J. Histopathology of alcohol-related liver diseases. Clin. Liver Dis. 2019, 23, 11–23. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Soliman, M.; Iftikhar, A.; Iftikhar, M.; Mostafa Aboelenin, S.; Wang, C. Protective effects of ellagic acid against alcoholic liver disease in mice. Front. Nutr. 2021, 8, 744520. [Google Scholar] [CrossRef]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Yuan, D.; Usman, M.; Murtaza, M.A.; Yaqoob, S.; Wang, C. Protective mechanism of edible food plants against alcoholic liver disease with special mention to polyphenolic compounds. Nutrients 2021, 13, 1612. [Google Scholar] [CrossRef]
- Allam, R.M.; Selim, D.A.; Ghoneim, A.I.; Radwan, M.M.; Nofal, S.M.; Khalifa, A.E.; Sharaf, O.A.; Toaima, S.M.; Asaad, A.M.; El-Sebakhy, N.A. Hepatoprotective effects of Astragalus kahiricus root extract against ethanol-induced liver apoptosis in rats. Chin. J. Nat. Med. 2013, 11, 354–361. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Luo, Y. Anti-aging implications of astragalus membranaceus (Huangqi): A well-known Chinese tonic. Aging Dis. 2017, 8, 868–886. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Han, X.; Xing, F.; Wu, H.; Shi, H.; Huang, F.; Xu, Q.; Wu, X. Total flavonoids of astragalus attenuates experimental autoimmune encephalomyelitis by suppressing the activation and inflammatory responses of microglia via JNK/AKT/NF kappa B signaling pathway. Phytomedicine 2021, 80, 153385. [Google Scholar] [CrossRef]
- Salehi, B.; Carneiro, J.N.P.; Rocha, J.E.; Coutinho, H.D.M.; Morais Braga, M.F.B.; Sharifi-Rad, J.; Semwal, P.; Painuli, S.; Moujir, L.M.; de Zarate Machado, V.; et al. Astragalus species: Insights on its chemical composition toward pharmacological applications. Phytother. Res. 2021, 35, 6974. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhang, Q.-Z.; Wang, Y.-R.; Fu, L.-N.; Han, J.-S.; Zhang, J.; Wang, B.-M. Astragaloside IV improves high-fat diet-induced hepatic steatosis in nonalcoholic fatty liver disease rats by regulating inflammatory factors level via TLR4/NF-kappa B signaling pathway. Front. Pharm. 2021, 11, 605064. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.L.; Tang, W.J.; Tang, B. Astragaloside IV inhibits protein tyrosine phosphatase 1B and improves insulin resistance in insulin-resistant HepG2 cells and triglyceride accumulation in oleic acid (OA)-treated HepG2 cells. J. Ethnopharmacol. 2021, 268, 113556. [Google Scholar] [CrossRef]
- Wang, E.; Wang, L.; Ding, R.; Zhai, M.; Ge, R.; Zhou, P.; Wang, T.; Fang, H.; Wang, J.; Huang, J. Astragaloside IV acts through multi-scale mechanisms to effectively reduce diabetic nephropathy. Pharm. Res. 2020, 157, 104831. [Google Scholar] [CrossRef]
- Hong, Y.; Li, B.; Zheng, N.; Wu, G.; Ma, J.; Tao, X.; Chen, L.; Zhong, J.; Sheng, L.; Li, H. Integrated metagenomic and metabolomic analyses of the effect of astragalus polysaccharides on alleviating high-fat diet-induced metabolic disorders. Front. Pharm. 2020, 11, 833. [Google Scholar] [CrossRef]
- Dong, N.; Li, X.; Xue, C.; Zhang, L.; Wang, C.; Xu, X.; Shan, A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-kappa B/MAPK signaling pathway. J. Cell Physiol. 2020, 235, 5525–5540. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef]
- Shen, X.; Sun, H.; Cui, H.; Jin, Y.; Jin, W.; Cui, X.; Guo, J. Astragaloside attenuates lipopolysaccharide-induced cell apoptosis in human gingiva cells via MAPK signaling pathway. J. Cell Biochem. 2019, 120, 12273–12279. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yin, L.; Sun, Z.; Shao, S.; Chen, W.; Man, X.; Du, Y.; Chen, Y. Astragalus polysaccharide exerts anti-Parkinson via activating the PI3K/AKT/mTOR pathway to increase cellular autophagy level in vitro. Int. J. Biol. Macromol. 2020, 153, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Motz, V.A.; Bowers, C.P.; Kneubehl, A.R.; Lendrum, E.C.; Young, L.M.; Kinder, D.H. Efficacy of the saponin component of Impatiens capensis Meerb.in preventing urushiol-induced contact dermatitis. J. Ethnopharmacol. 2015, 162, 163–167. [Google Scholar] [CrossRef]
- Rahman, E.; Momin, A.; Zhao, L.; Guo, X.; Xu, D.; Zhou, F.; Ji, B. Bioactive, nutritional composition, heavy metal and pesticide residue of four Chinese jujube cultivars. Food Sci. Biotechnol. 2018, 27, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, N.; Yang, D.; Yang, M.; Guo, X.; He, J.; Wu, W.; Ji, B.; Cheng, Q.; Zhou, F. Protective effects of five structurally diverse flavonoid subgroups against chronic alcohol-induced hepatic damage in a mouse model. Nutrients 2018, 10, 1754. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Hou, W.; Yao, P.; Zhang, B.; Sun, S.; Nuessler, A.K.; Liu, L. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol. In Vitro 2010, 24, 516–522. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, X.; Zhao, J. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-kappa B and STAT3 signaling pathway. Exp. Med. 2017, 14, 6169–6175. [Google Scholar]
- Sugimoto, K.; Takei, Y. Pathogenesis of alcoholic liver disease. Hepatol. Res. 2017, 47, 70–79. [Google Scholar] [CrossRef]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Han, C.; Wang, X.; Xing, G.; Zhou, L.; Li, G.; Niu, Y. Astragaloside IV suppresses collagen production of activated hepatic stellate cells via oxidative stress-mediated p38 MAPK pathway. Free Radic. Biol. Med. 2013, 60, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, X.; Zhang, Y.; Xu, J.; Zhang, L.; Li, Z.; Liu, W.; Ouyang, J.; Han, S.; He, X. Astragalus polysaccharide induces anti-inflammatory effects dependent on AMPK activity in palmitate-treated RAW264.7 cells. Int. J. Mol. Med. 2013, 31, 1463–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, T.M., Jr.; Osna, N.A.; Trambly, C.S.; Whitaker, N.P.; Thomes, P.G.; Todero, S.L.; Davis, J.S. Early growth response-1 contributes to steatosis development after acute ethanol administration. Alcohol. Clin. Exp. Res. 2012, 36, 759–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wang, L.; Zhang, H.; Zhang, D.; Zhang, Z.; Zhang, J. Protective effect of artemisinin on chronic alcohol induced-liver damage in mice. Environ. Toxicol. Pharm. 2017, 52, 221–226. [Google Scholar] [CrossRef]
- De Silva, N.M.G.; Borges, M.C.; Hingorani, A.D.; Engmann, J.; Shah, T.; Zhang, X.; Luan, J.a.; Langenberg, C.; Wong, A.; Kuh, D.; et al. Liver function and risk of type 2 diabetes: Bidirectional mendelian randomization study. Diabetes 2019, 68, 1681–1691. [Google Scholar] [CrossRef] [Green Version]
- Thapa, B.R.; Walia, A. Liver function tests and their interpretation. Indian J. Pediatr. 2007, 74, 663–671. [Google Scholar] [CrossRef]
- Zayed Mohamed, N.; Aly, H.F.; Moneim El-Mezayen, H.A.; El-Salamony, H.E. Effect of co-administration of Bee honey and some chemotherapeutic drugs on dissemination of hepatocellular carcinoma in rats. Toxicol. Rep. 2019, 6, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, G.; Kanbur, M.; Karabacak, M.; Arslan, K.; Silig, Y.; Soyer Sarica, Z.; Tekeli, M.Y.; Tas, A. Effect on oxidative stress, hepatic chemical metabolizing parameters, and genotoxic damage of mad honey intake in rats. Hum. Exp. Toxicol. 2018, 37, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Chen, B.; Chen, C.; Xu, J.; Shen, Z.; Miao, X.; Yao, H. Honey reduces blood alcohol concentration but not affects the level of serum MDA and GSH-Px activity in intoxicated male mice models. BMC Complementary Altern. Med. 2015, 15, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Xu, Q.; Hu, J.N.; Han, X.Y.; Li, W.; Zhao, L.C. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Kamel, R.; El Morsy, E.M. Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats. Arch. Pharm. Res. 2013, 36, 1140–1148. [Google Scholar] [CrossRef]
- Bahrami, A.; Parsamanesh, N.; Atkin, S.L.; Banach, M.; Sahebkar, A. Effect of statins on toll-like receptors: A new insight to pleiotropic effects. Pharm. Res. 2018, 135, 230–238. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-H.; Bang, J.; Son, C.-N.; Baek, W.-K.; Kim, J.-M. Grape seed proanthocyanidin extract ameliorates murine autoimmune arthritis through regulation of TLR4/MyD88/NF-kappa B signaling pathway. Korean J. Intern. Med. 2018, 33, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, B.; Jeon, K.; Moon, S.; Lee, K.; Kim, W.-K.; Jeong, H.; Cha, K.H.; Lim, M.Y.; Kang, W.; Kweon, M.-N.; et al. Roseburia spp. abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice. Cell Host Microbe 2020, 27, 25–40. [Google Scholar] [CrossRef]
- Ming, L.; Qiao, X.; Yi, L.; Siren, D.; He, J.; Hai, L.; Guo, F.; Xiao, Y.; Ji, R. Camel milk modulates ethanol-induced changes in the gut microbiome and transcriptome in a mouse model of acute alcoholic liver disease. J. Dairy Sci. 2020, 103, 3937–3949. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 mixture prevents chronic alcoholic liver injury in mice by protecting the intestinal barrier and regulating gut microbiota and liver-related pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of probiotic actions—A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]








| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Gadph | TCTCCTGCGACTTCAACA | TGTAGCCGTATTCATTGTCA |
| Keap1 | CAGATTGACAGCGTGGTT | GCAGTGTGACAGGTTGAA |
| Nfe2l2 | GTGCTCCTATGCGTGAAT | TCTTACCTCTCCTGCGTATA |
| Hmox1 | AGGTCCTGAAGAAGATTGC | TCTCCAGAGTGTTCATTCG |
| Nqo1 | ATGAAGGAGGCTGCTGTA | AGATGACTCGGAAGGATACT |
| Tlr4 | TGACATTCCTTCTTCAACCA | CACAGCCACCAGATTCTC |
| Myd88 | CCGTGAGGATATACTGAAGG | TTAAGCCGATAGTCTGTCTG |
| Nfkb1 | AGACAAGCAGCAGGACAT | CCAGCAACATCTTCACATC |
| Gadph | TCTCCTGCGACTTCAACA | TGTAGCCGTATTCATTGTCA |
| Groups | NG | MG | APL | APH | ASL | ASH |
|---|---|---|---|---|---|---|
| Initial weight (g) | 28.71 ± 1.46 | 28.47 ± 1.97 | 28.77 ± 1.32 | 28.71 ± 1.37 | 28.84 ± 1.48 | 28.66 ± 1.51 |
| Final weight (g) | 34.17 ± 3.11 | 29.68 ± 2.36 ** | 29.14 ± 1.50 | 30.05 ± 2.69 | 30.01 ± 1.39 | 29.50 ± 3.07 |
| Food intake (g/d) | 4.90 ± 0.46 | 3.63 ± 0.66 ** | 3.42 ± 0.70 | 3.53 ± 0.65 | 3.30 ± 0.75 | 3.73 ± 0.70 |
| Liver index (%) | 3.69 ± 0.36 | 3.90 ± 0.25 ** | 3.71 ± 0.26 # | 3.89 ± 0.15 | 4.10 ± 0.37 | 3.89 ± 0.19 |
| Kidney index (%) | 1.35 ± 0.12 | 1.46 ± 0.10 * | 1.44 ± 0.08 | 1.34 ± 0.10 # | 1.36 ± 0.08 | 1.34 ± 0.04 # |
| Spleen index (%) | 0.27 ± 0.04 | 0.31 ± 0.03 * | 0.27 ± 0.02 # | 0.26 ± 0.03 # | 0.27 ± 0.02 # | 0.26 ± 0.03 # |
| Testis index (%) | 0.70 ± 0.10 | 0.82 ± 0.07 * | 0.71 ± 0.04 # | 0.71 ± 0.07 # | 0.81 ± 0.06 | 0.85 ± 0.02 |
| Abdominal fat (%) | 0.24 ± 0.04 | 0.36 ± 0.08 ** | 0.26 ± 0.14 | 0.29 ± 0.17 | 0.32 ± 0.08 | 0.24 ± 0.07 # |
| Epididymal fat (%) | 1.13 ± 0.20 | 1.51 ± 0.27 ** | 1.20 ± 0.29 # | 1.16 ± 0.32 # | 1.39 ± 0.15 | 1.48 ± 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, N.; Zhao, L.; Wu, W.; Zhang, L.; Zhou, F.; Li, J. Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice. Foods 2021, 10, 2688. https://doi.org/10.3390/foods10112688
Zhou J, Zhang N, Zhao L, Wu W, Zhang L, Zhou F, Li J. Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice. Foods. 2021; 10(11):2688. https://doi.org/10.3390/foods10112688
Chicago/Turabian StyleZhou, Jingxuan, Nanhai Zhang, Liang Zhao, Wei Wu, Liebing Zhang, Feng Zhou, and Jingming Li. 2021. "Astragalus Polysaccharides and Saponins Alleviate Liver Injury and Regulate Gut Microbiota in Alcohol Liver Disease Mice" Foods 10, no. 11: 2688. https://doi.org/10.3390/foods10112688