Physicochemical Parameters, Phytochemical Profile and Antioxidant Properties of a New Beverage Formulated with Xique-Xique (Pilosocereus gounellei) Cladode Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Ingredients
2.2. Preparation of Xique-Xique, Passion Fruit and Lime Juice
2.3. Formulation of Beverages
2.4. Physical and Physicochemical Characterization of Formulated Beverages
2.5. Determination of Sugar and Organic Acid Profile of Formulated Beverages
2.6. Determination of Total Carotenoid Content in Formulated Beverages
2.7. Total Flavonoid and Total Phenolic Compound Contents in Formulated Beverages
2.8. Determination of Phenolic Compound Profile in Formulated Beverages
2.9. Evaluation of Antioxidant Activities of Formulated Beverages
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physical and Physicochemical Characteristics of Beverages
3.2. Soluble Sugar and Organic Acid Contents of Beverages
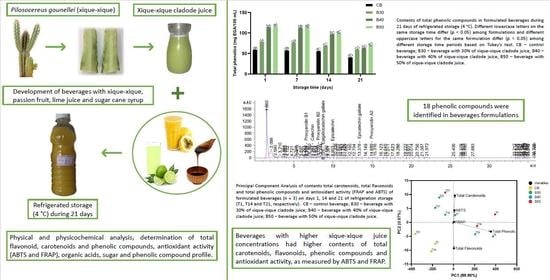
3.3. Total Contents of Carotenoids, Flavonoids and Phenolic Compounds of Beverages
3.4. Phenolic Compound Profile of Beverages
3.5. Antioxidant Activity of Beverages
3.6. Correlation between Antioxidant Activity and Bioactive Compounds in Beverages
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.D.; Faria, J.A.F. Functional foods and non-dairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-based alternatives to yogurt: State-of-the-art and perspectives of new biotechnological challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef]
- Nazir, M.; Arif, S.; Khan, R.S.; Nazir, W.; Khalid, N.; Maqsood, S. Opportunities and challenges for functional and medicinal beverages: Current and future trends. Trends Food Sci. Technol. 2019, 88, 513–526. [Google Scholar] [CrossRef]
- Market Research. Global Health Beverage Market—Analysis by Type of Beverage, by Sub-Types, by Sales Channel (Online, Offline), by Region, by Country. Available online: www.marketresearch.com/Food-Beverage-c84/Beverages-c165/ (accessed on 10 January 2021).
- Kausar, H.; Saeed, S.; Ahmad, M.M.; Salam, A. Studies on the development and storage stability of cucumber-melon functional drink. J. Agric. Res. 2012, 50, 239–248. [Google Scholar] [CrossRef]
- Bader-Ul-Ain, H.; Abbas, M.; Saeed, F.; Khalid, S.; Suleria, H.A.R. Functional nonalcoholic beverages: A global trend toward a healthy life. In Non-Alcohol. Beverages; Grumezescu, A., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Volume 6, Chapter 3; pp. 73–105. [Google Scholar]
- Gironés-Vilaplana, A.; Mena, P.; García-Viguera, C.; Moreno, D.A. A novel beverage rich in antioxidant phenolics: Maqui berry (Aristotelia chilensis) and lemon juice. LWT 2012, 47, 279–286. [Google Scholar] [CrossRef]
- Baccouche, A.; Ennouri, M.; Felfould, I.; Attia, H. A physical stability study of whey-based prickly pear beverage. Food Hydrocoll. 2013, 33, 234–244. [Google Scholar] [CrossRef]
- Mishra, L.K.; Sangma, D. Quality attributes, phytochemical profile and storage stability studies of functional ready to serve (RTS) drink made from blend of Aloe vera, sweet lime, amla and ginger. J. Food Sci. Technol. 2017, 54, 761–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciel, J.K.S.; Chavez, O.S.; Brito Filho, S.G.; Telles, Y.C.F.; Fernandes, M.G.; Assis, T.S.; Fernandes, P.D.; Andrade, A.P.; Felix, L.P.; Silva, T.M.S.; et al. New alcamide and anti-oxidant activity of Pilosocereus gounellei A. Weber ex K. Schum. Bly. ex Rowl. (Cactaceae). Molecules 2016, 21, 11. [Google Scholar] [CrossRef] [Green Version]
- Roque, A.A.; Rocha, R.M.; Loiola, M.I.B. Use and diversity of medicinal plants from Caatinga in the rural community of Laginhas, Caicó Municipality, Rio Grande do Norte State (Northeast of Brazil). Rev. Bras. Plant. Med. 2010, 12, 31–42. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Lucena, C.M.; Carvalho, T.K.; Ribeiro, J.E.; Quirino, Z.G.; Casas, A.; Lucena, R.F.P. Traditional botanical knowledge about the cactus in the semiarid of Brazil. Gaia Sci. 2015, 9, 77–90. (In Portuguese) [Google Scholar]
- Lucena, C.M.; Costa, G.M.; Sousa, R.F.; Carvalho, T.K.N.; Marreiros, N.A.; Alves, C.A.B.; Pereira, D.D.; Lucena, R.F.P. Local knowledge about cacti in the mesoregion of Paraíba State (Northeastern Brazil). Biotemas 2012, 25, 281–291. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, U.P.; Medeiros, P.M.; Almeida, A.L.S.; Monteiro, J.M.; Lins Neto, E.M.F.; Melo, J.G.; Santos, J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: A quantitative approach. J. Ethnopharmacol. 2007, 114, 325–354. [Google Scholar] [CrossRef]
- Almeida, C.A.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Oliveira, F.M.N. Physical and chemical characteristics of xiquexique pulps. Rev. Ciênc. Agron. 2007, 38, 440–443. (In Portuguese) [Google Scholar]
- Lucena, C.M.; Lucena, R.F.P.; Costa, G.M.; Carvalho, T.K.N.; Costa, G.G.S.; Alves, R.R.N.; Pereira, D.D.; Ribeiro, J.E.S.; Alves, C.A.B.; Quirino, Z.G.M.; et al. Use and knowledge of Cactaceae in Northeastern Brazil. J. Ethnobiol. Ethnomed. 2013, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.A.; Dantas, R.L.; Véras, C.S.; Alves, R.E.; Silva, S.M. Physical and physicochemical characteristics, bioactive compounds and antioxidant activity of xiquexique (Pilosocereus gounellei (A. Weber ex K. Schum.)) fruit. Semin. Ciênc. Agrár. 2018, 39, 1969–1980. [Google Scholar] [CrossRef]
- Ribeiro, T.S.; Sampaio, K.B.; Menezes, F.N.D.D.; Assis, P.O.A.; Lima, M.S.; Oliveira, M.E.G.; Souza, E.L.; Queiroga, R.C.R.E. In Vitro evaluation of potential prebiotic effects of a freeze-dried juice from Pilosocereus gounellei (A. Weber ex K. Schum. Bly. Ex Rowl) cladodes, an unconventional edible plant from Caatinga biome. 3 Biotech 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Assis, P.O.A.; Guerra, G.C.B.; Araújo, D.F.S.; Andrade, L.F.L.I.; Araújo, A.A.; Araújo Júnior, R.F.; Carvalho, T.G.; Souza, M.F.V.; Borges, G.S.C.; Lima, M.S. Intestinal anti-inflammatory activity of xique-xique (Pilosocereus gounellei A. Weber ex K. Schum. Bly. Ex Rowl) juice on acetic acid-induced colitis in rats. Food Funct. 2019, 10, 7275–7290. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Freire, M.O.L.; Silva, W.A.V.; Ferreira, M.R.A.; Paiva, P.M.G.; Soares, L.A.L.; Medeiros, P.L.; Carvalho, B.M.; Napoleão, T.H. Saline extract of Pilosocereus gounellei stem has antinociceptive effect in mice without showing acute toxicity and altering motor coordination. Regul. Toxicol. Pharmacol. 2018, 95, 289–297. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Silva, W.A.V.; Ferreira, M.R.A.; Paiva, P.M.G.; Medeiros, P.L.; Soares, L.A.L.; Carvalho, B.M.; Napoleão, T.H. Assessment of 28-day oral toxicity and antipyretic activity of the saline extract from Pilosocereus gounellei (Cactaceae) stem in mice. J. Ethnopharmacol. 2019, 234, 96–105. [Google Scholar] [CrossRef]
- Kieling, D.D.K.; Barbosa-Canóvas, G.V.; Prudencio, S.H. Effects of high pressure processing on the physicochemical and microbiological parameters, bioactive compounds and antioxidant activity of a lemongrass-lime mixed beverage. J. Food Sci. Technol. 2019, 56, 409–419. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Beserra, Y.A.S.; Oliveira, H.M.L.; Pasquali, M.A.D.B. Production of probiotic passion fruit (Passiflora edulis Sims f. Flavicarpa Deg.) drink using Lactobacillus reuteri and microencapsulation via spray drying. Foods 2020, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Maia, G.A.; Silva, L.M.R.; Prado, G.M.; Fonseca, A.V.V.; Sousa, P.H.M.; Figueiredo, R.W. Development of mixed beverages based on tropical fruits. In Non-Alcohol. Beverages; Grumezescu, A., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Volume 6, Chapter 5; pp. 129–162. [Google Scholar]
- Nogueira, F.S.; Ferreira, K.S.; Carneiro Júnior, J.B.; Passoni, L.C. Minerals in sugar cane syrup and cane juice. Ciênc. Tecnol. Aliment. 2009, 29, 727–731. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- A.O.A.C. Official Methods of Analysis of A.O.A.C. International, 20th ed.; AOAC International: Rockville, MD, USA, 2016; p. 3172. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Coelho, E.M.; Padilha, C.V.S.; Miskinis, G.A.; Sá, A.G.B.; Pereira, G.E.; Azevêdo, L.C.; Lima, M.S. Simultaneous analysis of sugars and organic acids in wine and grape juices by HPLC: Method validation and characterization of products from northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.Q.; Weber, C.; Lee, C.Y.; Brown, J.; Liu, R.H. Antioxidant and antiproliferative activities of raspberries. J. Agric. Food Chem. 2002, 50, 2926–2930. [Google Scholar] [CrossRef]
- Dutra, M.C.P.; Rodrigues, L.L.; Oliveira, D.; Pereira, G.E.; Lima, M.S. Integrated analyses of phenolic compounds and minerals of Brazilian organic and conventional grape juices and wines: Validation of a method for determination of Cu, Fe and Mn. Food Chem. 2018, 269, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Gonçalves, A.E.S.S.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Sariburun, E.; Sahin, S.; Demir, C.; Turkben, C.; Uylaser, V. Phenolic content and antioxidant activity of raspberry cultivars. J. Food Sci. 2010, 75, 328–335. [Google Scholar] [CrossRef]
- Rocha, I.F.O.; Bolini, H.M.A. Passion fruit juice with different sweeteners: Sensory profile by descriptive analysis and acceptance. Food Sci. Nutr. 2014, 3, 129–139. [Google Scholar] [CrossRef]
- Reis, L.C.R.; Facco, E.M.P.; Flôres, S.H.; Rios, A.O. Stability of functional compounds and antioxidant activity of fresh and pasteurized orange passion fruit (Passiflora caerulea) during cold storage. Food Res. Int. 2018, 106, 481–486. [Google Scholar] [CrossRef]
- Carvalho, P.O.A.A.; Guerra, G.C.B.; Borges, G.S.C.; Bezerril, F.F.; Sampaio, K.B.; Ribeiro, T.S.; Pacheco, M.T.B.; Milani, R.F.; Goldbeck, R.; Ávila, P.F. Nutritional potential and bioactive compounds of xique-xique juice: An unconventional food plant from Semiarid Brazilian. J. Food Process. Preserv. 2021, 45, 1–10. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; King, W.S.; Sahrir, M.A.S. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J. Sci. Food. Agric. 2012, 93, 1198–1205. [Google Scholar] [CrossRef]
- Schnurer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenols in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Wang, J.; Xie, B.; Sun, Z. Quality parameters and bioactive compound bioaccessibility changes in probiotics fermented mango juice using ultraviolet-assisted ultrasonic pre-treatment during cold storage. LWT 2021, 137, 110438. [Google Scholar] [CrossRef]
- Gonçalves, A.S.M.; Peixe, R.G.; Sato, A.; Muzitano, M.F.; Souza, R.O.M.A.; Machado, T.B.; Amaral, A.C.F.; Moura, M.R.L.; Simas, N.K.; Leal, I.C.R. Pilosocereus arrabidae (Byles & Rowley) of the Grumari Sandbank, RJ, Brazil: Physical, chemical characterizations and antioxidant activities correlated to detection of flavonoids. Food Res. Int. 2015, 70, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.M.C.; Alves, C.A.N.; Santos, K.L.; Silva, E.O.; Araújo, I.M.S.; Vasconcelos, L.B. Elaboration of a mixed beverage from hibiscus and coconut water: An evaluation of bioactive and sensory properties. Int. J. Gastron. Food Sci. 2021, 23, 100284. [Google Scholar] [CrossRef]
- Silveira, M.R.; Coutinho, N.M.; Esmerino, E.A.; Moraes, J.; Fernandes, L.M.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S. Guava-flavored whey beverage processed by cold plasma technology: Bioactive compounds, fatty acid profile and volatile compounds. Food Chem. 2019, 279, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.M.; Salvia-Trujillo, L.; Rojas-Grau, M.A.; Martín-Belloso, O. Changes on phenolic and carotenoid composition of high intensity pulsed electric field and thermally treated fruit juice-soymilk beverages during refrigerated storage. Food Chem. 2011, 129, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.B.; Araújo, F.P.; Neto, A.F.; Freitas, S.T.; Araújo, J.S.; Vilar, S.B.O.; Araújo, A.J.B.; Lima, M.S. Phytochemical compounds and antioxidant activity of the pulp of two Brazilian passion fruit species: Passiflora cincinnata Mast. and Passiflora Edulis Sims. Int. J. Fruit Sci. 2021, 21, 1–15. [Google Scholar] [CrossRef]
- Taeye, C.D.; Caullet, G.; Evina, V.J.E.; Collin, S. Procyanidin A2 and its degradation products in raw, fermented, and roasted cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Formulations 1 | |||
|---|---|---|---|---|
| CB | B30 | B40 | B50 | |
| Xique-xique juice | - | 30% | 40% | 50% |
| Passion fruit juice | 80% | 50% | 40% | 30% |
| Lime juice | 15% | 15% | 15% | 15% |
| Sugar cane syrup | 5% | 5% | 5% | 5% |
| Parameters | Time (Days) | Formulations | |||
|---|---|---|---|---|---|
| CB | B30 | B40 | B50 | ||
| pH | 1 | 3.14 ± 0.01 aA | 3.19 ± 0.00 aA | 3.19 ± 0.01 aA | 3.09 ± 0.01 aA |
| 7 | 2.93 ± 0.01 bB | 3.00 ± 0.01 abA | 3.01 ± 0.01 aAB | 2.99 ± 0.01 abAB | |
| 14 | 2.86 ± 0.00 aB | 2.92 ± 0.02 aAB | 2.95 ± 0.01 aBC | 2.97 ± 0.01 aBC | |
| 21 | 2.80 ± 0.00 bC | 2.90 ± 0.01 abB | 2.93 ± 0.01 aC | 2.91 ± 0.01 abC | |
| TA 1 (g/100 mL) | 1 | 11.66 ± 0.13 aB | 9.42 ± 0.08 bB | 8.66 ± 0.08 cB | 7.99 ± 0.13 dA |
| 7 | 12.21 ± 0.26 aAB | 9.59 ± 0.07 bAB | 8.83 ± 0.07 cAB | 7.90 ± 0.08 dA | |
| 14 | 12.47 ± 0.27 aAB | 10.27 ± 0.13 bA | 9.21 ± 0.19 cAB | 8.66 ± 0.29 cA | |
| 21 | 12.76 ± 0.27 aA | 10.10 ± 0.27 bAB | 9.51 ± 0.13 bA | 9.51 ± 0.49 bA | |
| TSS 2 (°Brix) | 1 | 6.90 ± 0.01 aB | 5.95 ± 0.05 bB | 6.10 ± 0.05 bB | 5.75 ± 0.05 cC |
| 7 | 7.40 ± 0.05 aB | 6.95 ± 0.05 bA | 6.20 ± 0.10 dB | 6.65 ± 0.05 cB | |
| 14 | 7.85 ± 0.05 aA | 7.00 ± 0.10 bA | 6.80 ± 0.05 bA | 6.80 ± 0.05 bB | |
| 21 | 8.00 ± 0.05 aA | 7.00 ± 0.10 cA | 6.95 ± 0.05 cA | 7.50 ± 0.05 bA | |
| Total ash (g/100 mL) | 1 | 0.26 ± 0.03 bA | 0.39 ± 0.01 aA | 0.40 ± 0.04 aAB | 0.47 ± 0.06 aAB |
| 7 | 0.21 ± 0.01 bA | 0.35 ± 0.04 aA | 0.39 ± 0.02 aAB | 0.42 ± 0.01 aAB | |
| 14 | 0.19 ± 0.03 bA | 0.31 ± 0.05 abA | 0.40 ± 0.02 aA | 0.38 ± 0.05 aA | |
| 21 | 0.16 ± 0.01 bA | 0.26 ± 0.06 abA | 0.31 ± 0.01 aB | 0.30 ± 0.03 aB | |
| Total sugars (g/100 mL) | 1 | 9.10 ± 0.10 aB | 7.97 ± 0.11 bD | 8.95 ± 0.10 aB | 8.31 ± 0.10 bB |
| 7 | 10.24 ± 0.06 aA | 8.87 ± 0.03 bC | 9.07 ± 0.12 bB | 8.98 ± 0.26 bAB | |
| 14 | 10.68 ± 0.27 aA | 9.12 ± 0.07 bB | 10.00 ± 0.18 aA | 9.24 ± 0.05 bA | |
| 21 | 10.70 ± 0.29 aA | 9.64 ± 0.10 bA | 10.34 ± 0.20 aA | 9.80 ± 0.43 abA | |
| Proteins (g/100 mL) | 1 | 0.58 ± 0.01 aA | 0.46 ± 0.01 bA | 0.46 ± 0.02 bcA | 0.41 ± 0.01 cA |
| 7 | 0.55 ± 0.02 aA | 0.49 ± 0.04 aA | 0.50 ± 0.03 aA | 0.45 ± 0.05 aA | |
| 14 | 0.51 ± 0.02 aA | 0.46 ± 0.06 aA | 0.45 ± 0.05 aA | 0.45 ± 0.06 aA | |
| 21 | 0.56 ± 0.05 aA | 0.49 ± 0.04 aA | 0.47 ± 0.07 aA | 0.46 ± 0.05 aA | |
| Lipids (g/100 mL) | 1 | 0.97 ± 0.14 aA | 0.67 ± 0.14 abA | 0.68 ± 0.18 abA | 0.42 ± 0.02 bA |
| 7 | 0.91 ± 0.10 aA | 0.77 ± 0.33 aA | 0.68 ± 0.33 aA | 0.48 ± 0.37 aA | |
| 14 | 0.99 ± 0.07 aA | 0.71 ± 0.11 abA | 0.62 ± 0.10 bA | 0.48 ± 0.29 abA | |
| 21 | 1.00 ± 0.01 aA | 0.80 ± 0.27 aA | 0.70 ± 0.35 aA | 0.48 ± 0.37 aA | |
| Parameters (g/100 mL) | Time (Days) | Beverages | |||
|---|---|---|---|---|---|
| CB | B30 | B40 | B50 | ||
| Soluble sugars | |||||
| Glucose | 1 | 1.00 ± 0.15 abA | 0.68 ± 0.07 abA | 0.55 ± 0.01 bB | 0.94 ± 0.01 aA |
| 14 | 1.03 ± 0.21 abA | 0.83 ± 0.02 bA | 0.98 ± 0.09 abAB | 1.05 ± 0.01 aA | |
| 21 | 1.05 ± 0.01 aA | 1.02 ± 0.11 aA | 1.02 ± 0.04 aA | 1.09 ± 0.03 aA | |
| Fructose | 1 | 0.87 ± 0.18 aA | 0.75 ± 0.07 aA | 0.67 ± 0.02 aB | 0.87 ± 0.01 aA |
| 14 | 0.97 ± 0.04 aA | 0.79 ± 0.03 aA | 0.82 ± 0.08 aAB | 0.90 ± 0.01 aA | |
| 21 | 1.04 ± 0.15 aA | 0.85 ± 0.09 aA | 0.98 ± 0.04 aA | 1.03 ± 0.04 aA | |
| Organic Acids | |||||
| Citric | 1 | 0.97 ± 0.14 aA | 0.83 ± 0.06 aA | 0.73 ± 0.05 aA | 0.72 ± 0.01 aA |
| 14 | 1.01 ± 0.09 abA | 0.84 ± 0.06 abA | 0.82 ± 0.01 aA | 0.74 ± 0.01 bA | |
| 21 | 1.12 ± 0.03 aA | 0.89 ± 0.02 bA | 0.82 ± 0.02 bA | 0.76 ± 0.01 cA | |
| Malic | 1 | 0.07 ± 0.02 aA | 0.07 ± 0.01 aA | 0.06 ± 0.01 aAB | 0.07 ± 0.00 aA |
| 14 | 0.06 ± 0.01 aA | 0.06 ± 0.02 aA | 0.04 ± 0.00 aB | 0.03 ± 0.00 aB | |
| 21 | 0.09 ± 0.00 aA | 0.09 ± 0.01 aA | 0.09 ± 0.00 aA | 0.06 ± 0.00 aA | |
| Succinic | 1 | 0.04 ± 0.01 aA | 0.03 ± 0.01 aA | 0.03 ± 0.00 aA | 0.02 ± 0.00 aA |
| 14 | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aB | |
| 21 | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.02 ± 0.00 aA | 0.01 ± 0.00 aAB | |
| Lactic | 1 | 0.01 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aAB | 0.02 ± 0.00 aA |
| 14 | 0.03 ± 0.02 aA | 0.03 ± 0.01 aA | 0.02 ± 0.00 aB | 0.02 ± 0.00 aA | |
| 21 | 0.02 ± 0.00 bA | 0.02 ± 0.00 bA | 0.03 ± 0.00 aA | 0.03 ± 0.00 aA | |
| Phenolic Compounds (mg/100 g) | Time (Days) | Beverages | |||
|---|---|---|---|---|---|
| CB | B30 | B40 | B50 | ||
| Flavanones | |||||
| Hesperidin | 1 | 0.08 ± 0.01 aA | 0.05 ± 0.00 aA | <LOD | <LOD |
| 14 | 0.07 ± 0.00 aA | 0.05 ± 0.00 aA | <LOD | <LOD | |
| 21 | 0.07 ± 0.01 aA | 0.05 ± 0.00 aA | <LOD | <LOD | |
| Naringenin | 1 | 0.02 ± 0.00 a | 0.02 ± 0.00 aA | 0.02 ± 0.00 a | 0.03 ± 0.00 a |
| 14 | <LOD | 0.02 ± 0.00 A | <LOD | <LOD | |
| 21 | <LOD | <LOD | <LOD | <LOD | |
| Flavonols | |||||
| Kaempferol | 1 | 0.13 ± 0.02 aA | 0.13 ± 0.00 aA | 0.13 ± 0.00 aA | 0.13 ± 0.00 aA |
| 14 | 0.13 ± 0.00 aA | 0.12 ± 0.01 aA | 0.13 ± 0.02 aAB | 0.13 ± 0.00 aA | |
| 21 | 0.13 ± 0.01 aA | 0.11 ± 0.01 aA | 0.12 ± 0.01 aB | 0.12 ± 0.01 aA | |
| Myricetin | 1 | 0.37 ± 0.05 aA | 0.35 ± 0.00 aA | 0.37 ± 0.06 aA | 0.36 ± 0.02 aA |
| 14 | 0.34 ± 0.01 aA | 0.32 ± 0.02 aA | 0.35 ± 0.02 aA | 0.36 ± 0.00 aA | |
| 21 | 0.33 ± 0.02 aA | 0.30 ± 0.01 aA | 0.33 ± 0.01 aA | 0.35 ± 0.00 aA | |
| Quercitin | 1 | 0.05 ± 0.00 aA | 0.04 ± 0.00 aA | 0.04 ± 0.00 aA | 0.04 ± 0.00 aA |
| 14 | 0.05 ± 0.00 aA | 0.05 ± 0.01 aA | 0.04 ± 0.00 aA | 0.04 ± 0.00 aA | |
| 21 | 0.04 ± 0.01 aA | 0.04 ± 0.00 aA | 0.04 ± 0.00 aA | 0.04 ± 0.00 aA | |
| Rutin | 1 | 0.03 ± 0.01 aA | 0.03 ± 0.02 aA | 0.04 ± 0.01 aA | 0.05 ± 0.00 aA |
| 14 | 0.03 ± 0.00 aA | 0.03 ± 0.02 aA | 0.03 ± 0.00 aA | 0.03 ± 0.00 aAB | |
| 21 | 0.02 ± 0.01 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aB | |
| Hydroxybenzoic acids | |||||
| Syringic acid | 1 | 0.03 ± 0.01 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.01 ± 0.00 aA |
| 14 | 0.03 ± 0.00 aA | 0.02 ± 0.00 abA | 0.02 ± 0.00 abA | 0.01 ± 0.00 bA | |
| 21 | 0.02 ± 0.00 aA | 0.02 ± 0.00 abA | 0.01 ± 0.00 bA | 0.01 ± 0.00 bA | |
| Hydroxynamic acids | |||||
| Caffeic acid | 1 | 0.02 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA |
| 14 | 0.02 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | |
| 21 | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | |
| Coumaric acid | 1 | 0.02 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.01 aA | 0.01 ± 0.00 aA |
| 14 | 0.02 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | |
| 21 | 0.02 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | 0.01 ± 0.00 aA | |
| Caftaric acid | 1 | 0.05 ± 0.01 aA | 0.05 ± 0.00 aA | 0.05 ± 0.00 aA | 0.05 ± 0.00 aA |
| 14 | 0.05 ± 0.00 aA | 0.05 ± 0.00 aA | 0.05 ± 0.02 aA | 0.05 ± 0.00 aA | |
| 21 | 0.03 ± 0.00 aA | 0.03 ± 0.00 aA | 0.04 ± 0.00 aA | 0.05 ± 0.00 aA | |
| Chlorogenic acid | 1 | 0.07 ± 0.00 abA | 0.07 ± 0.00 aA | 0.05 ± 0.01 abA | 0.04 ± 0.00 bA |
| 14 | 0.07 ± 0.01 aAB | 0.05 ± 0.00 aA | 0.04 ± 0.01 aA | 0.04 ± 0.00 aA | |
| 21 | 0.06 ± 0.00 aB | 0.05 ± 0.00 aA | 0.04 ± 0.00 abA | 0.04 ± 0.00 bA | |
| Polyphenols | |||||
| Trans-resveratrol | 1 | 0.02 ± 0.00 aA | 0.02 ± 0.00 aAB | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA |
| 14 | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | |
| 21 | 0.02 ± 0.00 aA | 0.01 ± 0.00 aB | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | |
| Cis-resveratrol | 1 | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA |
| 14 | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | |
| 21 | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | |
| Epicatechins gallate | 1 | 0.17 ± 0.00 aA | 0.11 ± 0.00 abA | 0.10 ± 0.00 bA | 0.08 ± 0.00 cA |
| 14 | 0.16 ± 0.02 aA | 0.10 ± 0.00 aB | 0.09 ± 0.00 aA | 0.06 ± 0.01 aAB | |
| 21 | 0.15 ± 0.01 aA | 0.09 ± 0.03 abAB | 0.07 ± 0.01 abA | 0.05 ± 0.00 bB | |
| Epicatechins | 1 | 0.04 ± 0.01 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA | 0.02 ± 0.00 aA |
| 14 | 0.04 ± 0.00 aA | 0.02 ± 0.00 abA | 0.02 ± 0.00 bA | 0.02 ± 0.00 bB | |
| 21 | 0.03 ± 0.00 aA | 0.02 ± 0.00 aA | 0.01 ± 0.00 bA | 0.01 ± 0.00 bB | |
| Flavanols | |||||
| Epigallocatechin gallate | 1 | 0.25 ± 0.05 aA | 0.16 ± 0.00 abA | 0.15 ± 0.03 abA | 0.12 ± 0.00 bA |
| 14 | 0.23 ± 0.01 aAB | 0.14 ± 0.01 bAB | 0.14 ± 0.01 bA | 0.11 ± 0.01 bA | |
| 21 | 0.18 ± 0.01 aB | 0.11 ± 0.01 abB | 0.12 ± 0.03 abA | 0.09 ± 0.02 bA | |
| Anthocyanins | |||||
| Procyanidin B1 | 1 | 0.06 ± 0.01 aA | 0.05 ± 0.00 aA | 0.06 ± 0.01 aA | 0.05 ± 0.00 aA |
| 14 | 0.05 ± 0.01 aA | 0.04 ± 0.00 aA | 0.05 ± 0.00 aA | 0.05 ± 0.00 aA | |
| 21 | 0.05 ± 0.00 aA | 0.04 ± 0.00 aA | 0.05 ± 0.00 aA | 0.05 ± 0.00 aA | |
| Procyanidin A2 | 1 | 0.13 ± 0.00 cA | 0.14 ± 0.00 bA | 0.14 ± 0.00 bA | 0.15 ± 0.00 aA |
| 14 | 0.13 ± 0.00 bAB | 0.13 ± 0.01 abA | 0.14 ± 0.2 abAB | 0.14 ± 0.00 aB | |
| 21 | 0.12 ± 0.01 bB | 0.12 ± 0.00 bB | 0.13 ± 0.00 aB | 0.13 ± 0.00 aC | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, J.M.C.; de Souza, E.L.; de Lima, K.Y.G.; dos S. Lima, M.; Viera, V.B.; Queiroga, R.d.C.R.d.E.; de Oliveira, M.E.G. Physicochemical Parameters, Phytochemical Profile and Antioxidant Properties of a New Beverage Formulated with Xique-Xique (Pilosocereus gounellei) Cladode Juice. Foods 2021, 10, 1970. https://doi.org/10.3390/foods10091970
de Oliveira JMC, de Souza EL, de Lima KYG, dos S. Lima M, Viera VB, Queiroga RdCRdE, de Oliveira MEG. Physicochemical Parameters, Phytochemical Profile and Antioxidant Properties of a New Beverage Formulated with Xique-Xique (Pilosocereus gounellei) Cladode Juice. Foods. 2021; 10(9):1970. https://doi.org/10.3390/foods10091970
Chicago/Turabian Stylede Oliveira, Julia M. C., Evandro L. de Souza, Kaíque Y. G. de Lima, Marcos dos S. Lima, Vanessa B. Viera, Rita de Cássia R. do E. Queiroga, and Maria Elieidy G. de Oliveira. 2021. "Physicochemical Parameters, Phytochemical Profile and Antioxidant Properties of a New Beverage Formulated with Xique-Xique (Pilosocereus gounellei) Cladode Juice" Foods 10, no. 9: 1970. https://doi.org/10.3390/foods10091970