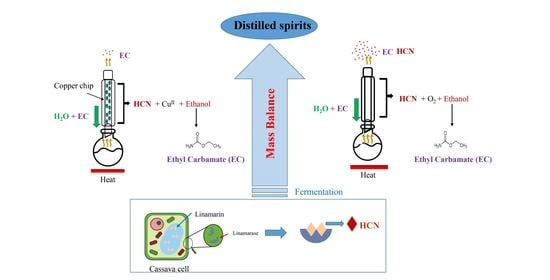
Effect of Fermentation on Cyanide and Ethyl Carbamate Contents in Cassava Flour and Evaluation of Their Mass Balance during Lab-Scale Continuous Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Ethanol Fermentation from Cassava Flour
2.3. Lab-Scale Continuous Distillation
2.4. Pre-Distillation for Cyanide Measurement
2.5. Cyanide Determination by Colorimetric Methods
2.6. Effect of Ethanol on the Measurement of Cyanide
2.7. Cyanide Determination by Ion Chromatography
2.8. Ethyl Carbamate Measurement by GC-MS Method
2.9. Mass Balance of Cyanide and Ethyl Carbamate during Continuous Distillation
2.10. Reduction of Cyanide by Copper Chips
2.11. Identification of Linamarin by Liquid Chromatography-Mass Spectrometry (LC-MS)
3. Results and Discussion
3.1. Reduction of Cyanide during Ethanol Fermentation
3.2. Total Change in Cyanide Content during the Lab-Scale Continuous Distillation with and without Copper Chips
3.3. Formation of Ethyl Carbamate during Ethanol Fermentation and Its Migration during Lab-Scale Continuous Distillation with or without Copper Chips
3.4. Presence of Linamarin in Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO/IFAD. The World Cassava Economy: Facts, Trends and Outlooks; Food and Agriculture Organization of the United Nations and International Fund for Agricultural Development: Rome, Italy, 2000. [Google Scholar]
- Breuninger, W.F.; Piyachomkwan, K.; Sriroth, K. Chapter 12—Tapioca/Cassava starch: Production and use. In Starch, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Hahn, S.K. An overview of African traditional cassava processing and utilization. Outlook Agric. 1989, 18, 110–118. [Google Scholar] [CrossRef]
- Inlow, D.; McRae, J.; Ben-Bassat, A. Fermentation of corn starch to ethanol with genetically engineered yeast. Biotechnol. Bioeng. 1988, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kobayashi, F.; Ohnaga, M.; Sawada, T. Alcohol fermentation of starch by a genetic recombinant yeast having glucoamylase activity. Biotechnol. Bioeng. 1997, 53, 21–25. [Google Scholar] [CrossRef]
- Akintonwa, A.; Tunwashe, O.; Onifade, A. Fatal and non-fatal acute poisoning attributed to cassava-based meal. Acta Hortic. 1994, 375, 285–288. [Google Scholar] [CrossRef]
- Cliff, J.; Coutinho, J. Acute intoxication from newly-introduced cassava during drought in Mozambique. Trop. Doct. 1995, 25, 193. [Google Scholar] [CrossRef]
- Gruhnert, C.; Biehl, B.; Selmar, D. Compartmentation of cyanogenic glucosides and their degrading enzymes. Planta 1994, 195, 36–42. [Google Scholar] [CrossRef]
- McMahon, J.M.; White, W.L.B.; Sayre, R.T. Cyanogenesis in cassava (Manihot esculenta Crantz). J. Exp. Bot. 1995, 46, 731–741. [Google Scholar] [CrossRef]
- Beasley, D.M.G.; Glass, W.I. Cyanide poisoning: Pathophysiology and treatment recommendations. Occup. Med. 1998, 7, 427–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, K.; Buhrke, T.; Lampen, A. Bioavailability of cyanide after consumption of a single meal of foods containing high levels of cyanogenic glycosides: A cross over study in humans. Arch. Toxicol. 2016, 90, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Siritunga, D.; Sayre, R.T. Generation of cyanogen-free transgenic cassava. Planta 2003, 217, 367–373. [Google Scholar] [CrossRef]
- Dufour, D.L. Cyanide content of cassava (Manihot esculenta Euphorbiaceae) cultivars used by Tukanoan Indians in northwest Amazonia. Econ. Bot. 1988, 42, 255–266. [Google Scholar] [CrossRef]
- FAO/WHO. Codex Standard for Edible Cassava Flour. Codex Standard 176-1989; Food and Agriculture Organization and World Health Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- Cardoso, A.P.; Mirione, E.; Ernesto, M.; Massaza, F.; Cliff, J.; Haque, M.R.; Bradbury, J.H. Processing of cassava roots to remove cyanogens. J. Food Compos. Anal. 2005, 18, 451–460. [Google Scholar] [CrossRef]
- Dennis, M.J.; Howarth, N.; Key, P.E.; Pointer, M.; Massey, R.C. Investigation of ethyl carbamate levels in some fermented foods and alcoholic beverages. Food Addit. Contam. 1989, 6, 383–389. [Google Scholar] [CrossRef]
- Beland, F.A.; Benson, R.W.; Mellick, P.W.; Kovatch, R.M.; Roberts, D.W.; Fang, J.L.; Doerge, D.R. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food Chem. Toxicol. 2005, 43, 1–19. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0278691504002479 (accessed on 29 March 2021). [CrossRef] [PubMed]
- European Food Safety Authority. Ethyl carbamate and hydrocyanic acid in food and beverages. EFSA J. 2007, 551, 1–44. [Google Scholar]
- Lim, H.S.; Lee, K.G. Development and validation of analytical methods for ethyl carbamate in various fermented foods. Food Chem. 2011, 126, 1373–1379. [Google Scholar] [CrossRef]
- Aylott, R.I.; Cochrane, G.C.; Leonard, M.J.; Macdonald, L.S.; Mackenzie, W.M.; Mcneish, A.S.; Walker, D.A. Ethyl carbamate formation in grain based spirits. Part I: Post-distillation ethyl carbamate formation in maturing grain whisky. J. Inst. Brew. 1990, 96, 213–221. [Google Scholar] [CrossRef]
- Mendonça, J.G.P.; Cardoso, M.D.G.; Santiago, W.D.; Rodrigues, L.M.A.; Nelson, D.L.; Brandão, R.M.; Silva, L. Determination of ethyl carbamate in cachaças produced by selected yeast and spontaneous fermentation. J. Inst. Brew. 2016, 122, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Riffkin, H.L.; Wilson, R.; Howie, D.; Muller, S.B. Ethyl carbamate formation in the production of pot still whisky. J. Inst. Brew. 1989, 95, 115–119. [Google Scholar] [CrossRef]
- Santiago, W.D.; Cardoso, M.D.G.; Duarte, F.C.; Saczk, A.A.; Nelson, D.L. Ethyl carbamate in the production and aging of cachaça in oak (Quercus sp.) and amburana (Amburana cearensis) barrels. J. Inst. Brew. 2014, 120, 507–511. [Google Scholar] [CrossRef]
- Aresta, M.; Boscolo, M.; Franco, D.W. Copper (II) catalysis in cyanide conversion into ethyl carbamate in spirits and relevant reactions. J. Agric. Food Chem. 2001, 49, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Galinaro, C.; Ohe, T.H.K.; da Silva, A.C.H.; da Silva, S.C.; Franco, D.W. Cyanate as an active precursor of ethyl carbamate formation in sugar can spirit. J. Agric. Food Chem. 2015, 63, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- MOE. Official Test Methods of Soil Contamination. In Cyanide-Ultraviolet/Visible Spectrometry. ES 07352.1a; Ministry of Environment: Sejong, Korea, 2009. [Google Scholar]
- NHFPC. National Health and Family Planning Commission of PRC. National Standard of the People’s Republic of China: National Food Safety Standard-Determination of Cyanide in Foods. GB 5009.36–2016; NHFPC: Beijing, China, 2016. [Google Scholar]
- König, W. Untersuchungen aus dem organischen Laboratorium der Technischen Hochschule zu Dresden. J. Prakt. Chem. 1904, 69, 105–137. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.L.; Ramasamy, J.; Paukstelis, J.V. Stable reagents for the colorimetric determination of cyanide by modified König reactions. Anal. Chem. 1975, 47, 916–918. [Google Scholar] [CrossRef]
- Izatt, R.M.; Christensen, J.J.; Pack, R.T.; Bench, R. Thermodynamics of metal–cyanide coordination: I. pK, △H0, △S0 values as a function of temperature for hydrocyanic acid dissociation in aqueous solutions. Inorg. Chem. 1962, 1, 828–831. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International. In Guidelines for Standard Method Performance Requirements, 19th ed.; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]
- Mackenzie, W.M.; Clyne, A.H.; Macdonald, L.S. Ethyl Carbamate Formation in Grain Based Spirits. Part II: The Identification and Determination of Cyanide Related Species Involved in Ethyl Carbamate Formation in Scotch Grain Whisky. J. Inst. Brew. 1990, 96, 223–232. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/j.2050-0416.1990.tb01031.x (accessed on 29 March 2021). [CrossRef]
- WHO. International Agency for Research on Cancer. In IARC Monographs on the Identification of Carcinogenic Hazards to Humans. No. 96; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Tham, M.T. Distillation–an introduction/Factors Affecting Distillation Column Operation. Available online: https://www.rccostello.com/distil/distilop.htm (accessed on 29 March 2021).
- Zuk, M.; Pelc, K.; Szperlik, J.; Sawula, A.; Szopa, J. Metabolism of the Cyanogenic Glucosides in Developing Flax: Metabolic Analysis, and Expression Pattern of Genes. Metabolites 2020, 10, 288. [Google Scholar] [CrossRef]
- Nartey, F. Studies on cassava, Manihot utilissima Pohl—I. Cyanogenesis: The biosynthesis of linamarin and lotaustralin in etiolated seedlings. Phytochemistry 1968, 8, 1307–1312. [Google Scholar] [CrossRef]


| Samples 1 | I-P 2 | P-P 3 | IC 4 | Average 5 | RSD 6 (%) | Reduction (%) |
|---|---|---|---|---|---|---|
| Cassava flour | 0.43 ± 0.07 | 0.43 ± 0.11 | 0.35 ± 0.09 | 0.40 ± 0.05 | 11.2 | 0 |
| Steaming | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.11 ± 0.01 | 7.6 | 72.6 |
| Saccharification | 0.09 ± 0.00 | 0.09 ± 0.00 | 0.09 ± 0.01 | 0.09 ± 0.00 | 1.8 | 78.6 |
| Fermentation | 0.09 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.01 | 14.1 | 81.5 |
| Spiked CN− Concentration | Measured CN− Concentration | RR 1 (%) | |
|---|---|---|---|
| Absence of copper chips | 5.00 | 5.60 ± 0.00 | 112.0 |
| Presence of copper chips | 5.00 | 1.91 ± 0.56 | 34.1 |
| After ethanol evaporation 2 | 0.06 | 0.06 ± 0.00 | 98.5 |
| 0.15 | 0.14 ± 0.01 | 92.3 | |
| 0.30 | 0.29 ± 0.02 | 94.9 | |
| Before ethanol evaporation 3 | 0.06 | 0.08 ± 0.04 | >100 (132.2) |
| 0.15 | 0.22 ± 0.01 | >100 (147.8) | |
| 0.30 | 0.41 ± 0.03 | >100 (135.1) |
| Cyanide Content 1 | DSMR 2 (%) | DSRR 3 (%) | RR 4 (%) | Copper Chips | |
|---|---|---|---|---|---|
| Fermented liquid (pre-distillate) | 0.56 ± 0.03 | ||||
| Fermented liquid (filtrate) | 0.60 ± 0.02 | ||||
| Distilled spirits (ethanol evaporation) | 0.53 ± 0.04 | 88.3 | 93.3 | – 6 | |
| Distillers’ stillage (filtrate) | 0.03 ± 0.00 | 5.0 | |||
| Distilled spirits (ethanol evaporation) | ND 5 | 0 | 13.3 | + 7 | |
| Distillers’ stillage (filtrate) | 0.08 ± 0.01 | 13.3 | |||
| Copper chips | detected | ||||
| Samples | Ethyl Carbamate Content | DSMR 1 (%) | DSRR 2 (%) | RR 3 (%) | Copper Chips |
|---|---|---|---|---|---|
| Ethanol fermentation process 4 | |||||
| Raw material (pre-distillate) | ND 5 | ||||
| Steaming (pre-distillate) | Trace 6 | ||||
| Saccharification (pre-distillate) | Trace | ||||
| Fermentation (pre-distillate) | 0.16 ± 0.01 | ||||
| Continuous distillation process | |||||
| Fermented liquid (pre-distillate) | 14.42 ± 1.41 | ||||
| Fermented liquid (filtrate) | 0.34 ± 0.01 | ||||
| Distilled spirits | 10.55 ± 0.02 | 73.2 | 90.1 | – 7 | |
| Distillers’ stillage (filtrate) | 2.44 ± 0.07 | 16.9 | |||
| Distilled spirits | 1.39 ± 1.76 | 9.6 | 107.9 | + 8 | |
| Distillers’ stillage (filtrate) | 14.17 ± 2.05 | 98.3 | |||
| Copper chips | Detected | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Duan, B.; Shin, J.-A.; So, H.-J.; Hong, E.-S.; Jeong, H.-G.; Lee, J.-H.; Lee, K.-T. Effect of Fermentation on Cyanide and Ethyl Carbamate Contents in Cassava Flour and Evaluation of Their Mass Balance during Lab-Scale Continuous Distillation. Foods 2021, 10, 1089. https://doi.org/10.3390/foods10051089
Qin Y, Duan B, Shin J-A, So H-J, Hong E-S, Jeong H-G, Lee J-H, Lee K-T. Effect of Fermentation on Cyanide and Ethyl Carbamate Contents in Cassava Flour and Evaluation of Their Mass Balance during Lab-Scale Continuous Distillation. Foods. 2021; 10(5):1089. https://doi.org/10.3390/foods10051089
Chicago/Turabian StyleQin, Yan, Beibei Duan, Jung-Ah Shin, Hee-Jin So, Eun-Sik Hong, Hee-Gon Jeong, Jeung-Hee Lee, and Ki-Teak Lee. 2021. "Effect of Fermentation on Cyanide and Ethyl Carbamate Contents in Cassava Flour and Evaluation of Their Mass Balance during Lab-Scale Continuous Distillation" Foods 10, no. 5: 1089. https://doi.org/10.3390/foods10051089