Reduction of Polycyclic Aromatic Hydrocarbons (PAHs) in Sesame Oil Using Cellulosic Aerogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
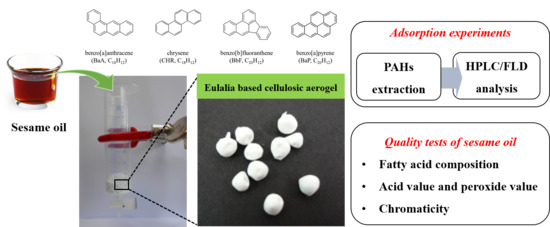
2.2. Preparation of Cellulosic Aerogel
2.3. Preparation of Sesame Seed Oil
2.4. Extraction and Purification of PAHs
2.5. Analysis of Polycyclic Aromatic Hydrocarbons (PAHs)
2.6. Quality Analysis of Sesame Seed Oil
2.6.1. Analysis of Fatty Acid Composition
2.6.2. Analysis of Chemical Properties
2.6.3. Chromaticity
2.7. Statistical Analysis
3. Results and Discussion
3.1. PAHs Adsorption of Eulalia-Based Cellulosic Aerogel
3.2. Quality Properties of Sesame Oil
3.2.1. Analysis of Fatty Acid Content of Sesame Oils
3.2.2. Analysis of the Chemical Characteristics
3.2.3. Chromaticity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food–A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Penning, T. Processing contaminants: Polycyclic aromatic hydrocarbons (PAHs). In Encyclopedia of Food Safety, 1st ed.; Motarjemi, Y., Moy, G., Todd, E., Eds.; Academic Press: Waltham, MA, USA, 2014; Volume 2, pp. 416–423. [Google Scholar]
- Sánchez-Arévalo, C.M.; Olmo-García, L.; Fernández-Sánchez, J.F.; Carrasco-Pancorbo, A. Polycyclic aromatic hydrocarbons in edible oils: An overview on sample preparation, determination strategies, and relative abundance of prevalent compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3528–3573. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.; Kumari, R.; Bhat, K. Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn. Rev. 2014, 8, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namayandeh, S.M.; Kaseb, F.; Lesan, S. Olive and sesame oil effect on lipid profile in hypercholesterolemic patients, which better? Int. J. Prev. Med. 2013, 4, 1059. [Google Scholar]
- Kaur, I.P.; Saini, A. Sesamol exhibits antimutagenic activity against oxygen species mediated mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 470, 71–76. [Google Scholar] [CrossRef]
- IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 92, 754–759. [Google Scholar]
- Commission, E. Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, 215, 1–5. [Google Scholar]
- Kim, D.-Y.; Han, G.-T.; Shin, H.-S. Adsorption of polycyclic aromatic hydrocarbons (PAHs) by cellulosic aerogels during smoked pork sausage manufacture. Food Control 2021, 124. [Google Scholar] [CrossRef]
- Yi, J.Y.; Kim, H.J.; Chung, M.-S. Manufacture of low-benzo (a) pyrene sesame seed (Sesamum indicum L.) oil using a self-designed apparatus. PLoS ONE 2017, 12, e0173585. [Google Scholar] [CrossRef] [Green Version]
- Beh, J.H.; Lim, T.H.; Lew, J.H.; Lai, J.C. Cellulose nanofibril-based aerogel derived from sago pith waste and its application on methylene blue removal. Int. J. Biol. Macromol. 2020, 160, 836–845. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohydr. Polym. 2021, 251, 117016. [Google Scholar] [CrossRef]
- Ji, Y.; Wen, Y.; Wang, Z.; Zhang, S.; Guo, M. Eco-friendly fabrication of a cost-effective cellulose nanofiber-based aerogel for multifunctional applications in Cu (II) and organic pollutants removal. J. Clean. Prod. 2020, 255, 120276. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, flexible, and superhydrophobic functionalized cellulose aerogel for selective and versatile oil/water separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Lin, R.; Li, A.; Zheng, T.; Lu, L.; Cao, Y. Hydrophobic and flexible cellulose aerogel as an efficient, green and reusable oil sorbent. RSC Adv. 2015, 5, 82027–82033. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A robust salt-tolerant superoleophobic chitosan/nanofibrillated cellulose aerogel for highly efficient oil/water separation. Carbohydr. Polym. 2018, 200, 611–615. [Google Scholar] [CrossRef]
- Hajeeth, T. Adsorption of Cr (VI) from aqueous solutions using cellulose extracted from sisal fiber. Indian J. Appl. Res. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Li, N.; Bai, R. Copper adsorption on chitosan–cellulose hydrogel beads: Behaviors and mechanisms. Sep. Purif. Technol. 2005, 42, 237–247. [Google Scholar] [CrossRef]
- Lee, S.W.; Jeung, M.K.; Park, M.H.; Lee, S.Y.; Lee, J. Effects of roasting conditions of sesame seeds on the oxidative stability of pressed oil during thermal oxidation. Food Chem. 2010, 118, 681–685. [Google Scholar] [CrossRef]
- AOCS. Method Ce 2-66. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; AOCS Press: Champaign, IL, USA, 1997. [Google Scholar]
- AOCS. Method Cd 3d-63. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; AOCS Press: Champaign, IL, USA, 1997. [Google Scholar]
- AOCS. Method Cd 8-53. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; AOCS Press: Champaign, IL, USA, 1997. [Google Scholar]
- Kim, H.-Y.; Song, D.-S. Minimizing benzo (a) pyrene content in the manufacturing of sesame oil and perilla oil. Korean J. Food Preserv. 2008, 15, 556–561. [Google Scholar]
- Kang, C.-H.; Park, J.-K.; Park, J.-U.; Chun, S.-S.; Lee, S.-C.; Ha, J.-U.; Hwang, Y.-I. Comparative studies on the fatty acid composition of Korean and Chinese sesame oils and adulterated sesame oils with commercial edible oils. J. Korean Soc. Food. Sci. Nutr. 2002, 31, 17–20. [Google Scholar]
- Codex Alimentarius. Standard for Named Vegetable Oils, CXS 210-1999 Adopted in 1999. Revised in 2001, 2003, 2009, 2017. Amended in 2005, 2011, 2013, 2015. Codex Standard. 2017, pp. 1–13. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 17 March 2021).
- Ministry of Food and Drug Safety. Korean Food Standard Codex; Ministry of Food and Drug Safety: Cheongju-si, Chungcheongbuk-do, Korea, 2014.
- Ji, J.; Liu, Y.; Shi, L.; Wang, N.; Wang, X. Effect of roasting treatment on the chemical composition of sesame oil. LWT 2019, 101, 191–200. [Google Scholar] [CrossRef]
- Borchani, C.; Besbes, S.; Blecker, C.; Attia, H. Chemical characteristics and oxidative stability of sesame seed, sesame paste, and olive oils. J. Agric. Sci. Technol. 2010, 12, 585–596. [Google Scholar]
- Dim, P.; Adebayo, S.; Musa, J. Extraction and characterization of oil from sesame seed. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 752–757. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioproc. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]




| PAH 1 | Calibration Curve Equation | LOD (μg/kg) | LOQ (μg/kg) | R2 | Recovery (%) |
|---|---|---|---|---|---|
| BaA | y = 0.1074x − 0.0155 | 0.12 | 0.37 | 0.9993 | 88.58 ± 1.52 |
| CHR | y = 0.1086x − 0.0263 | 0.15 | 0.45 | 0.9959 | 91.07 ± 0.85 |
| BbF | y = 0.1009x + 0.003 | 0.15 | 0.46 | 0.9999 | 89.02 ± 1.29 |
| BaP | y = 0.3325x − 0.0142 | 0.15 | 0.45 | 0.9999 | 92.49 ± 0.55 |
| PAH 1 (μg/kg) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roasting | BaA | CHR | BbF | BaP | PAH4 | |||||||||||
| Temp. (°C) | Time (min) | Control | Cellulosic Aerogel | t-Value | Control | Cellulosic Aerogel | t-Value | Control | Cellulosic Aerogel | t-Value | Control | Cellulosic Aerogel | t-Value | Control | Cellulosic Aerogel | t-Value |
| 150 | 10 | 0.93 ± 0.01 bc | 0.64 ± 0.01 c | 35.10 *** | 0.41 ± 0.01 c | 0.30 ± 0.02 d | 10.18 *** | 0.18 ± 0.00 b | ND2 | - | 0.18 ± 0.00 b | ND | - | 1.71 ± 0.02 bB | 0.94 ± 0.01 dB | 48.98 *** |
| 20 | 0.91 ± 0.02 c | 0.64 ± 0.01 c | 19.98 *** | 0.42 ± 0.01 b | 0.27 ± 0.03 b | 7.73 ** | 0.17 ± 0.01 d | ND | - | 0.19 ± 0.01 b | ND | - | 1.69 ± 0.01 dB | 0.92 ± 0.04 dB | 36.47 *** | |
| 30 | 0.95 ± 0.02 c | 0.71 ± 0.01 d | 20.18 *** | 0.44 ± 0.02 d | 0.30 ± 0.01 d | 10.49 *** | 0.18 ± 0.01 c | ND | - | 0.19 ± 0.00 c | ND | - | 1.77 ± 0.03 cA | 1.01 ± 0.01 dA | 37.56 *** | |
| 180 | 10 | 0.91 ± 0.02 cB | 0.65 ± 0.01 bB | 17.82 *** | 0.46 ± 0.02 bC | 0.34 ± 0.02 cB | 7.97 ** | 0.19 ± 0.01 b | ND | - | 0.19 ± 0.01 ab | ND | - | 1.75 ± 0.00 bC | 1.00 ± 0.01 cC | 92.17 *** |
| 20 | 0.92 ± 0.01 cB | 0.66 ± 0.02 cB | 19.21 *** | 0.48 ± 0.01 bB | 0.30 ± 0.01 bC | 28.47 *** | 0.19 ± 0.01 c | 0.15 ± 0.01 | 4.42 * | 0.19 ± 0.01 b | ND | - | 1.78 ± 0.00 cB | 1.12 ± 0.03 cB | 40.60 *** | |
| 30 | 0.97 ± 0.02 cA | 0.76 ± 0.02 cA | 12.54 *** | 0.56 ± 0.01 cA | 0.42 ± 0.01 cA | 20.66 *** | 0.22 ± 0.02 c | 0.16 ± 0.01 | 5.26 ** | 0.20 ± 0.01 c | ND | - | 1.95 ± 0.02 bA | 1.34 ± 0.03 cA | 27.97 *** | |
| 210 | 10 | 1.13 ± 0.03 aB | 0.79 ± 0.01 aB | 16.98 *** | 0.47 ± 0.00 bB | 0.39 ± 0.01 bC | 9.76 *** | 0.21 ± 0.01 bC | 0.17 ± 0.01 | 5.35 ** | 0.20 ± 0.01 abB | ND | - | 2.00 ± 0.04 aC | 1.35 ± 0.02 aC | 24.48 *** |
| 20 | 1.13 ± 0.01 bB | 0.73 ± 0.02 bC | 41.72 *** | 0.59 ± 0.08 aA | 0.49 ± 0.01 aA | 2.12 | 0.25 ± 0.01 bB | 0.20 ± 0.01 B | 10.09 *** | 0.22 ± 0.02 aA | ND | - | 2.20 ± 0.06 bB | 1.43 ± 0.00 bB | 21.63 ** | |
| 30 | 1.80 ± 0.05 aA | 0.89 ± 0.01 aA | 30.76 *** | 0.62 ± 0.01 bA | 0.44 ± 0.01 bB | 21.06 *** | 0.30 ± 0.04 bA | 0.24 ± 0.01 A | 2.73 | 0.24 ± 0.01 bA | ND | - | 2.97 ± 0.05 aA | 1.58 ± 0.01 bA | 43.54 *** | |
| 240 | 10 | 0.97 ± 0.01 bC | 0.63 ± 0.01 cC | 34.20 *** | 0.53 ± 0.03 aC | 0.42 ± 0.01 aB | 5.67 * | 0.31 ± 0.02 aC | 0.20 ± 0.01 C | 7.33 ** | 0.20 ± 0.00 aB | ND | - | 2.02 ± 0.04 aC | 1.26 ± 0.03 bC | 27.48 *** |
| 20 | 1.27 ± 0.02 aB | 0.98 ± 0.02 aA | 18.17 *** | 0.61 ± 0.01 aB | 0.45 ± 0.03 aB | 10.01 | 0.45 ± 0.00 aB | 0.38 ± 0.01 B | 10.18 *** | 0.24 ± 0.01 aA | ND | - | 2.58 ± 0.02 aB | 1.82 ± 0.05 aB | 22.58 *** | |
| 30 | 1.30 ± 0.01 bA | 0.83 ± 0.02 bB | 43.52 *** | 0.72 ± 0.01 aA | 0.50 ± 0.01 aA | 26.30 *** | 0.55 ± 0.02 aA | 0.41 ± 0.01 A | 9.76 *** | 0.35 ± 0.02 aA | 0.22 ± 0.01 | 12.95 *** | 2.92 ± 0.03 aA | 1.97 ± 0.00 aA | 46.83 *** |
| Fatty Acid (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roasting | 16:0 | 18:0 | 18:1 | 18:2 | 18:3n3 | ||||||
| Temp. (°C) | Time (min) | Control | Cellulosic Aerogel | Control | Cellulosic Aerogel | Control | Cellulosic Aerogel | Control | Cellulosic Aerogel | Control | Cellulosic Aerogel |
| 150 | 10 | 9.30 ± 0.03 bA | 9.28 ± 0.00 aA | 5.26 ± 0.03 bA | 5.26 ± 0.00 aA | 39.46 ± 0.05 d | 39.45 ± 0.01 cA | 45.44 ± 0.03 bC | 44.93 ± 0.00 aB | 0.44 ± 0.01 dC | 0.42 ± 0.00 cC |
| 20 | 8.89 ± 0.01 cC | 8.37 ± 0.00 cC | 5.04 ± 0.01 bC | 3.44 ± 0.00 dC | 39.43 ± 0.03 b | 39.22 ± 0.00 cB | 45.96 ± 0.04 aA | 44.32 ± 0.00 aC | 0.58 ± 0.01 aB | 0.53 ± 0.00 bA | |
| 30 | 9.07 ± 0.01 bB | 9.01 ± 0.00 aB | 5.21 ± 0.00 bB | 4.30 ± 0.01 cB | 39.45 ± 0.01 b | 39.12 ± 0.01 cC | 45.58 ± 0.01 bB | 45.76 ± 0.04 bA | 0.59 ± 0.01 abA | 0.48 ± 0.01 cB | |
| 180 | 10 | 9.36 ± 0.00 aA | 8.29 ± 0.01 cC | 5.28 ± 0.01 abA | 3.43 ± 0.04 dC | 41.63 ± 0.01 aA | 41.05 ± 0.01 aA | 43.01 ± 0.02 dC | 43.61 ± 0.02 cC | 0.60 ± 0.01 aA | 0.50 ± 0.01 aB |
| 20 | 9.08 ± 0.01 bB | 8.76 ± 0.00 aB | 5.03 ± 0.01 bC | 4.66 ± 0.01 cB | 39.35 ± 0.05 cC | 39.54 ± 0.00 bB | 45.84 ± 0.02 bB | 44.33 ± 0.00 aB | 0.58 ± 0.01 aB | 0.57 ± 0.00 aA | |
| 30 | 9.06 ± 0.01 bC | 9.01 ± 0.01 aA | 5.04 ± 0.01 cB | 4.98 ± 0.01 aA | 39.35 ± 0.02 cB | 39.31 ± 0.01 bC | 45.84 ± 0.00 aA | 45.80 ± 0.01 aA | 0.59 ± 0.01 bB | 0.58 ± 0.01 bA | |
| 210 | 10 | 9.16 ± 0.01 cA | 8.85 ± 0.01 bA | 5.21 ± 0.01 cB | 5.15 ± 0.01 bA | 39.50 ± 0.00 cB | 39.32 ± 0.01 dB | 45.47 ± 0.01 aA | 44.20 ± 0.01 bA | 0.55 ± 0.00 cB | 0.37 ± 0.01 dB |
| 20 | 8.30 ± 0.01 dC | 8.00 ± 0.01 dC | 4.96 ± 0.00 cC | 4.78 ± 0.00 bB | 39.41 ± 0.04 bC | 39.21 ± 0.01 dC | 44.48 ± 0.01 cB | 43.27 ± 0.01 bC | 0.58 ± 0.01 aA | 0.47 ± 0.01 cA | |
| 30 | 9.08 ± 0.01 bB | 8.82 ± 0.01 bB | 5.37 ± 0.03 aA | 3.61 ± 0.01 dC | 40.72 ± 0.01 aA | 40.33 ± 0.01 aA | 44.35 ± 0.01 dC | 43.93 ± 0.01 dB | 0.46 ± 0.01 cC | 0.38 ± 0.00 dB | |
| 240 | 10 | 9.36 ± 0.01 aA | 8.23 ± 0.00 dB | 5.31 ± 0.02 aB | 4.37 ± 0.00 cC | 40.61 ± 0.01 bB | 40.81 ± 0.00 bB | 44.03 ± 0.01 cB | 43.01 ± 0.01 dC | 0.58 ± 0.01 bB | 0.47 ± 0.01 bB |
| 20 | 9.30 ± 0.01 aB | 8.52 ± 0.01 bA | 5.72 ± 0.01 aA | 4.92 ± 0.05 aA | 41.42 ± 0.01 aA | 41.05 ± 0.01 aA | 43.11 ± 0.01 dC | 43.08 ± 0.02 cB | 0.34 ± 0.01 bC | 0.31 ± 0.01 dC | |
| 30 | 9.12 ± 0.01 aC | 8.22 ± 0.01 cB | 4.95 ± 0.00 dC | 4.81 ± 0.01 bB | 38.81 ± 0.04 dC | 37.94 ± 0.00 dC | 44.82 ± 0.01 cA | 44.75 ± 0.01 cA | 0.60 ± 0.00 aA | 0.88 ± 0.01 aA | |
| t-value | 6.01 *** | 6.45 *** | 1.09 NS | 2.54 NS | 1.61 NS |
| Color | |||||||
|---|---|---|---|---|---|---|---|
| Roasting | L* | a* | b* | ||||
| Temp. (°C) | Time (min) | Control | Cellulosic Aerogel | Control | Cellulosic Aerogel | Control | Cellulosic Aerogel |
| 150 | 10 | 94.72 ± 0.01 cA | 95.47 ± 0.02 bA | −1.86 ± 0.01 aA | −2.22 ± 0.02 bB | 6.94 ± 0.01 dC | 6.65 ± 0.01 dC |
| 20 | 90.63 ± 0.01 aC | 88.84 ± 0.01 bC | −1.90 ± 0.02 cB | −1.64 ± 0.03 bA | 8.53 ± 0.01 dA | 8.78 ± 0.03 dB | |
| 30 | 91.37 ± 0.01 aB | 90.05 ± 0.06 aB | −1.83 ± 0.02 bA | −1.66 ± 0.02 bA | 7.96 ± 0.02 dB | 8.89 ± 0.02 dA | |
| 180 | 10 | 91.16 ± 0.01 dA | 92.19 ± 0.05 dA | −1.85 ± 0.02 aA | −1.82 ± 0.01 aA | 8.58 ± 0.01 cC | 8.49 ± 0.01 cC |
| 20 | 89.93 ± 0.03 bB | 90.14 ± 0.02 aB | −1.84 ± 0.00 bA | −1.98 ± 0.00 cB | 10.09 ± 0.07 cB | 9.77 ± 0.02 cB | |
| 30 | 87.21 ± 0.01 bC | 88.51 ± 0.07 bC | −2.22 ± 0.01 cB | −2.12 ± 0.02 cC | 11.22 ± 0.03 cA | 11.09 ± 0.05 cA | |
| 210 | 10 | 95.73 ± 0.02 aA | 96.06 ± 0.04 aA | −2.83 ± 0.01 bC | −2.92 ± 0.01 cC | 10.12 ± 0.01 bC | 10.06 ± 0.02 bC |
| 20 | 84.26 ± 0.03 cB | 85.34 ± 0.02 cB | −2.75 ± 0.02 dB | −2.80 ± 0.02 dB | 18.64 ± 0.01 bB | 18.31 ± 0.02 bB | |
| 30 | 45.67 ± 0.02 cC | 46.87 ± 0.05 cC | 0.06 ± 0.00 aA | 0.05 ± 0.01 aA | 20.66 ± 0.05 bA | 20.08 ± 0.02 bA | |
| 240 | 10 | 95.10 ± 0.02 bA | 94.57 ± 0.02 cA | −3.17 ± 0.01 cB | −3.07 ± 0.05 dB | 12.43 ± 0.11 aC | 12.15 ± 0.10 aC |
| 20 | 81.85 ± 0.04 dB | 82.11 ± 0.02 dB | 2.60 ± 0.03 aA | 2.63 ± 0.02 aA | 22.46 ± 0.05 aB | 21.10 ± 0.06 aB | |
| 30 | 20.54 ± 0.02 dC | 22.00 ± 0.06 dC | −3.21 ± 0.03 dC | −3.19 ± 0.01 dC | 52.59 ± 0.09 aA | 52.14 ± 0.10 aA | |
| t-value | −0.06 NS | −0.01 NS | 0.08 NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Kim, B.; Shin, H.-S. Reduction of Polycyclic Aromatic Hydrocarbons (PAHs) in Sesame Oil Using Cellulosic Aerogel. Foods 2021, 10, 644. https://doi.org/10.3390/foods10030644
Kim D-Y, Kim B, Shin H-S. Reduction of Polycyclic Aromatic Hydrocarbons (PAHs) in Sesame Oil Using Cellulosic Aerogel. Foods. 2021; 10(3):644. https://doi.org/10.3390/foods10030644
Chicago/Turabian StyleKim, Do-Yeong, Boram Kim, and Han-Seung Shin. 2021. "Reduction of Polycyclic Aromatic Hydrocarbons (PAHs) in Sesame Oil Using Cellulosic Aerogel" Foods 10, no. 3: 644. https://doi.org/10.3390/foods10030644