Nickel Hydroxide Nanofluid Cathodes with High Solid Loadings and Low Viscosity for Energy Storage Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization Techniques
2.2. Synthesis of Nickel Hydroxide Nanoparticles
2.3. Surface Modification of Nickel Hydroxide Nanoparticles
2.4. Preparation of Nanofluids Using Dry Nanopowders
2.5. Measurements of Nanofluid Properties
2.6. Electrochemical Testing
3. Results
3.1. Surface Modification of Nickel Hydroxide Nanoparticles
3.2. Rheology of Nickel Hydroxide Nanofluids
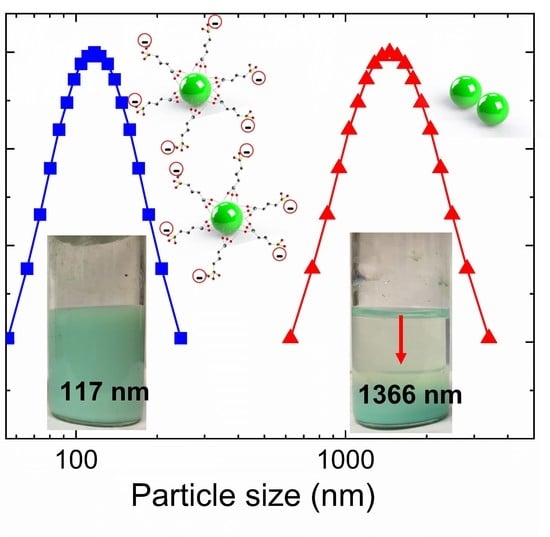
3.3. Colloidal Stability of Nanofluids
3.4. Thermal Conductivity of Nanofluids
3.5. Electrochemical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.; Townsend, J.; Christianson, R.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.W.; Alvarado, J.L.; et al. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef] [Green Version]
- Cingarapu, S.; Singh, D.; Timofeeva, E.V.; Moravek, M.R. Nanofluids with encapsulated tin nanoparticles for advanced heat transfer and thermal energy storage. Int. J. Energy Res. 2014, 38, 51–59. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Timofeeva, E.V.; Singh, D. Effective Thermal Conductivity Models for Carbon Nanotube-Based Nanofluids. J. Nanofluids 2013, 2, 69–73. [Google Scholar] [CrossRef]
- Raja, M.; Vijayan, R.; Dineshkumar, P.; Venkatesan, M. Review on nanofluids characterization, heat transfer characteristics and applications. Renew. Sustain. Energy Rev. 2016, 64, 163–173. [Google Scholar] [CrossRef]
- Babita; Sharma, S.K.; Gupta, S.M. Preparation and evaluation of stable nanofluids for heat transfer application: A review. Exp. Therm. Fluid Sci. 2016, 79, 202–212. [Google Scholar]
- Akhavan-Behabadi, M.A.; Shahidi, M.; Aligoodarz, M.R.; Fakoor-Pakdaman, M. An experimental investigation on rheological properties and heat transfer performance of MWCNT-water nanofluid flow inside vertical tubes. Appl. Therm. Eng. 2016, 106, 916–924. [Google Scholar] [CrossRef]
- Rueda-García, D.; Rodríguez-Laguna, M.d.R.; Chávez-Angel, E.P.; Dubal, D.; Cabán-Huertas, Z.; Benages-Vilau, R.; Gómez-Romero, P. From Thermal to Electroactive Graphene Nanofluids. Energies 2019, 12, 4545. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Garcia, D.; Caban-Huertas, Z.; Sanchez-Ribot, S.; Marchante, C.; Benages, R.; Dubal, D.P.; Ayyad, O.; Gomez-Romero, P. Battery and supercapacitor materials in flow cells. Electrochemical energy storage in a LiFePO4/reduced graphene oxide aqueous nanofluid. Electrochim. Acta 2018, 281, 594–600. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. Electroactive graphene nanofluids for fast energy storage. 2D Mater. 2016, 3, 031004. [Google Scholar] [CrossRef]
- Dubal, D.P.; Rudea-Garcia, D.; Marchante, C.; Benages, R.; Gomez-Romero, P. Hybrid Graphene-Polyoxometalates Nanofluids as Liquid Electrodes for Dual Energy Storage in Novel Flow Cells. Chem. Rec. 2018, 18, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.R.; Gonçalves, I.M.; Rodrigues, R.O.; Minas, G.; Miranda, J.M.; Moreira, A.L.N.; Lima, R.; Coutinho, G.; Pereira, J.E.; Moita, A.S. Recent advances on the thermal properties and applications of nanofluids: From nanomedicine to renewable energies. Appl. Therm. Eng. 2022, 201, 117725. [Google Scholar] [CrossRef]
- Liu, C.; Qiao, Y.; Du, P.; Zhang, J.; Zhao, J.; Liu, C.; Huo, Y.; Qi, C.; Rao, Z.; Yan, Y. Recent advances of nanofluids in micro/nano scale energy transportation. Renew. Sustain. Energy Rev. 2021, 149, 111346. [Google Scholar] [CrossRef]
- Gang, Q.; Wang, R.-T.; Wang, J.-C. Estimations on Properties of Redox Reactions to Electrical Energy and Storage Device of Thermoelectric Pipe (TEP) Using Polymeric Nanofluids. Polymers 2021, 13, 1812. [Google Scholar] [CrossRef] [PubMed]
- Aberoumand, S.; Woodfield, P.; Shabani, B.; Dao, D.V. Advances in electrode and electrolyte improvements in vanadium redox flow batteries with a focus on the nanofluidic electrolyte approach. Phys. Rep. 2020, 881, 1–49. [Google Scholar] [CrossRef]
- Bharathidasan, P.; Subramaniam, T.; Chandini, D.; Sivakkumar, S.R.; Rajan, K.S.; Devaraj, S. Simultaneous Enhancement of Energy and Power Density of Reduced Graphene Oxide by the Effect of Dispersed Metal Oxide Nanoparticles in the Electrolyte. J. Electrochem. Soc. 2020, 167, 140524. [Google Scholar] [CrossRef]
- Joseph, A.; Xavier, M.M.; Fal, J.; Żyła, G.; Sasi, S.; Radhakrishnan Nair, P.; Padmanabhan, A.S.; Mathew, S. Synthesis and electrochemical characterization of electroactive IoNanofluids with high dielectric constants from hydrated ferrous sulphate. J. Chem. Soc. Chem. Commun. 2019, 55, 83–86. [Google Scholar] [CrossRef]
- Kim, J.; Park, H. Synergistic effect of nanofluid as catalyst with carbon foam electrode for improved rheological properties of aqueous electrolytes for vanadium redox flow battery. J. Power Sources 2021, 500, 229974. [Google Scholar] [CrossRef]
- Said, Z.; Allagui, A.; Abdelkareem, M.A.; Alawadhi, H.; Elsaid, K. Acid-functionalized carbon nanofibers for high stability, thermoelectrical and electrochemical properties of nanofluids. J. Colloid Interface Sci. 2018, 520, 50–57. [Google Scholar] [CrossRef]
- Seed, C.M.; Acharya, B.; Perelygin, V.; Smirnov, A.I.; Krim, J. Tribotronic control and cyclic voltammetry of platinum interfaces with metal oxide nanofluids. Appl. Surf. Sci. 2021, 566, 150675. [Google Scholar] [CrossRef]
- Zapata-Hernandez, C.; Durango-Giraldo, G.; López, D.; Buitrago-Sierra, R.; Cacua, K. Surfactants versus surface functionalization to improve the stability of graphene nanofluids. J. Dispers. Sci. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Losev, A.V.; Petrii, O.A.; Nauki, I. Suspension and fluidized electrodes. Electrokhimiya 1979, 14, 120. [Google Scholar]
- Aberoumand, S.; Woodfield, P.; Shi, G.; Kien Nguyen, T.; Nguyen, H.-Q.; Li, Q.; Shabani, B.; Viet Dao, D. Thermo-electro-rheological behaviour of vanadium electrolyte-based electrochemical graphene oxide nanofluid designed for redox flow battery. J. Mol. Liq. 2021, 338, 116860. [Google Scholar] [CrossRef]
- Jung Lee, H.; Bai, S.-J.; Seok Song, Y. Microfluidic Electrochemical Impedance Spectroscopy of Carbon Composite Nanofluids. Sci. Rep. 2017, 7, 722. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Subudhi, S. Developments in fuel cells and electrochemical batteries using nanoparticles and nanofluids. Energy Storage 2021, 4, e288. [Google Scholar] [CrossRef]
- Sokolov, S.V.; Kätelhön, E.; Compton, R.G. Near-Wall Hindered Diffusion in Convective Systems: Transport Limitations in Colloidal and Nanoparticulate Systems. J. Phys. Chem. C 2016, 120, 10629–10640. [Google Scholar] [CrossRef]
- Sen, S.; Chow, C.-M.; Moazzen, E.; Segre, C.U.; Timofeeva, E.V. Electroactive nanofluids with high solid loading and low viscosity for rechargeable redox flow batteries. J. Appl. Electrochem. 2017, 47, 593–605. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox flow batteries for the storage of renewable energy: A review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Katsoudas, J.P.; Segre, C.U.; Singh, D. Rechargeable Nanofluid Electrodes for High Energy Density Flow Battery, Cleantech 2013, Chapter 9, Energy Storage. pp. 363–366. Available online: https://briefs.techconnect.org/wp-506content/volumes/Cleantech2013/pdf/535.pdf (accessed on 22 June 2022).
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137. [Google Scholar] [CrossRef] [Green Version]
- Garche, J.; Dietz, H.; Wiesener, K. The suspension electrode technique for electrochemical analysis of lead dioxide. J. Electroanal. Chem. Interfacial Electrochem. 1984, 180, 577–585. [Google Scholar] [CrossRef]
- Duduta, M.; Ho, B.; Wood, V.C.; Limthongkul, P.; Brunini, V.E.; Carter, W.C.; Chiang, Y.-M. Semi-Solid Lithium Rechargeable Flow Battery. Adv. Energy Mater. 2011, 1, 511–516. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.C.; Dong, Y.; Baram, N.; Fan, F.Y.; Xie, J.; Limthongkul, P.; Carter, W.C.; Chiang, Y.-M. Aqueous semi-solid flow cell: Demonstration and analysis. Phys. Chem. Chem. Phys. 2013, 15, 15833–15839. [Google Scholar] [CrossRef] [PubMed]
- Boota, M.; Hatzell, K.B.; Alhabeb, M.; Kumbur, E.C.; Gogotsi, Y. Graphene-containing flowable electrodes for capacitive energy storage. Carbon 2015, 92, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Hatzell, K.B.; Fan, L.; Beidaghi, M.; Boota, M.; Pomerantseva, E.; Kumbur, E.C.; Gogotsi, Y. Composite Manganese Oxide Percolating Networks as a Suspension Electrode for an Asymmetric Flow Capacitor. ACS Appl. Mater. Interfaces 2014, 6, 8886–8893. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhang, H.; Yang, L.; Xing, C.; Pan, S.; Lu, W.; Zhang, S. Effects of conductive additives on the percolation networks and rheological properties of LiMn0.7Fe0.3PO4 suspensions for lithium slurry battery. Chem. Eng. J. 2022, 433, 133203. [Google Scholar] [CrossRef]
- Wilk, J.; Grosicki, S. Limiting current technique in the research of mass/heat transfer in nanofluid. J. Phys. Conf. Ser. 2016, 745, 032084. [Google Scholar] [CrossRef] [Green Version]
- Meethong, N.; Huang, H.-Y.S.; Carter, W.C.; Chiang, Y.-M. Size-Dependent Lithium Miscibility Gap in Nanoscale Li1−xFePO4. Electrochem. Solid-State Lett. 2007, 10, A134. [Google Scholar] [CrossRef]
- Sen, S.; Moazzen, E.; Aryal, S.; Segre, C.U.; Timofeeva, E.V. Engineering nanofluid electrodes: Controlling rheology and electrochemical activity of γ-Fe2O3 nanoparticles. J. Nanopart. Res. 2015, 17, 437. [Google Scholar] [CrossRef]
- Sen, S.; Govindarajan, V.; Pelliccione, C.J.; Wang, J.; Miller, D.J.; Timofeeva, E.V. Surface Modification Approach to TiO2 Nanofluids with High Particle Concentration, Low Viscosity, and Electrochemical Activity. ACS Appl. Mater. Interfaces 2015, 7, 20538–20547. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel hydroxides and related materials: A review of their structures, synthesis and properties. Proc. R. Soc. A 2015, 471, 20140792. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B.; Liu, J.; Chen, G.; Gao, R.; Yao, S.; Li, M.; Zhang, Q.; Gu, L.; Xie, J.; et al. Oxide-Modified Nickel Photocatalysts for the Production of Hydrocarbons in Visible Light. Angew. Chem. Int. Ed. 2016, 55, 4215–4219. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient Water Oxidation Using Nanostructured α-Nickel-Hydroxide as an Electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, L.; Yan, Y.; Che, R.; Chen, M.; Wu, L. One-Step Fabrication of Ultrathin Porous Nickel Hydroxide-Manganese Dioxide Hybrid Nanosheets for Supercapacitor Electrodes with Excellent Capacitive Performance. Adv. Energy Mater. 2013, 3, 1636–1646. [Google Scholar] [CrossRef]
- Singu, B.S.; Male, U.; Hong, S.E.; Yoon, K.R. Synthesis and performance of nickel hydroxide nanodiscs for redox supercapacitors. Ionics 2016, 22, 1485–1491. [Google Scholar] [CrossRef]
- Mao, L.; Guan, C.; Huang, X.; Ke, Q.; Zhang, Y.; Wang, J. 3D Graphene-Nickel Hydroxide Hydrogel Electrode for High-Performance Supercapacitor. Electrochim. Acta 2016, 196, 653–660. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, C.; Tao, S.; Chu, W.; Wu, Z.; Li, Y. Ultrathin Nickel Hydroxide and Oxide Nanosheets: Synthesis, Characterizations and Excellent Supercapacitor Performances. Sci. Rep. 2014, 4, 5787. [Google Scholar] [CrossRef] [PubMed]
- Parveen, N.; Cho, M.H. Self-Assembled 3D Flower-Like Nickel Hydroxide Nanostructures and Their Supercapacitor Applications. Sci. Rep. 2016, 6, 27318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sialvi, M.Z.; Mortimer, R.J.; Wilcox, G.D.; Teridi, A.M.; Varley, T.S.; Wijayantha, K.G.U.; Kirk, C.A. Electrochromic and Colorimetric Properties of Nickel(II) Oxide Thin Films Prepared by Aerosol-Assisted Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2013, 5, 5675–5682. [Google Scholar] [CrossRef] [Green Version]
- Sonavane, A.C.; Inamdar, A.I.; Shinde, P.S.; Deshmukh, H.P.; Patil, R.S.; Patil, P.S. Efficient electrochromic nickel oxide thin films by electrodeposition. J. Alloys Compod. 2010, 489, 667–673. [Google Scholar] [CrossRef]
- Fantini, M.; Gorenstein, A. Electrochromic nickel hydroxide films on transparent/conducting substrates. Sol. Energy Mater. 1987, 16, 487–500. [Google Scholar] [CrossRef]
- Wen, R.-T.; Niklasson, G.A.; Granqvist, C.G. Electrochromic nickel oxide films and their compatibility with potassium hydroxide and lithium perchlorate in propylene carbonate: Optical, electrochemical and stress-related properties. Thin Solid Films 2014, 565, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.; Sandhyarani, N. A highly sensitive electrochemical glucose sensor structuring with nickel hydroxide and enzyme glucose oxidase. Electrochim. Acta 2013, 108, 274–280. [Google Scholar] [CrossRef]
- Canevari, T.C.; Cincotto, F.H.; Landers, R.; Machado, S.A.S. Synthesis and characterization of α-nickel (II) hydroxide particles on organic-inorganic matrix and its application in a sensitive electrochemical sensor for vitamin D determination. Electrochim. Acta 2014, 147, 688–695. [Google Scholar] [CrossRef]
- Yang, H.; Gao, G.; Teng, F.; Liu, W.; Chen, S.; Ge, Z. Nickel Hydroxide Nanoflowers for a Nonenzymatic Electrochemical Glucose Sensor. J. Electrochem. Soc. 2014, 161, B216–B219. [Google Scholar] [CrossRef]
- Gangwar, J.; Dey, K.K.; Tripathi, S.K.; Wan, M.; Yadav, R.R.; Singh, R.K.; Samta; Srivastava, A.K. NiO-based nanostructures with efficient optical and electrochemical properties for high-performance nanofluids. Nanotechnology 2013, 24, 415705. [Google Scholar] [CrossRef]
- Qazi, S.J.S.; Rennie, A.R.; Cockcroft, J.K.; Vickers, M. Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles. J. Colloid Interface Sci. 2009, 338, 105–110. [Google Scholar] [CrossRef]
- Qazi, S.J.S.; Rennie, A.R.; Tucker, I.; Penfold, J.; Grillo, I. Impact of Ni(OH)2 Platelike Particles on Lamellar Surfactant Mesophases and the Orientation of Their Mixtures under Elongational Flow. J. Phys. Chem. B 2011, 115, 10413–10424. [Google Scholar] [CrossRef]
- Qazi, S.J.S.; Karlsson, G.; Rennie, A.R. Self-Assembled Structures of Disc-Like Colloidal Particles. In Trends in Colloid and Interface Science XXIV; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Brown, A.B.D.; Clarke, S.M.; Rennie, A.R. Ordered Phase of Platelike Particles in Concentrated Dispersions. Langmuir 1998, 14, 3129–3132. [Google Scholar] [CrossRef]
- Brown, A.B.D.; Ferrero, C.; Narayanan, T.; Rennie, A.R. Phase separation and structure in a concentrated colloidal dispersion of uniform plates. Eur. Phys. J. B 1999, 11, 481–489. [Google Scholar] [CrossRef]
- Utomo, A.T.; Poth, H.; Robbins, P.T.; Pacek, A.W. Experimental and theoretical studies of thermal conductivity, viscosity and heat transfer coefficient of titania and alumina nanofluids. Int. J. Heat Mass Transfer 2012, 55, 7772–7781. [Google Scholar] [CrossRef]
- Using The Krieger−Dougherty Model to Predict Suspension, Viscosity. Technical Note, Version 5, Malvern Instruments. Available online: http://www.malvern.com/ (accessed on 22 June 2022).
- Routbort, J.L.; Singh, D.; Timofeeva, E.V.; Yu, W.; France, D.M. Pumping power of nanofluids in a flowing system. J. Nanopart. Res. 2011, 13, 931–937. [Google Scholar] [CrossRef]
- Slack, G.A.; Newman, R. Thermal Conductivity of MnO and NiO. Phys. Rev. Lett. 1958, 1, 359–360. [Google Scholar] [CrossRef]
- Keem, J.E.; Honig, J.M. Selected Electrical and Thermal Properties of Undoped Nickel Oxide. 1978, 578. Available online: https://apps.dtic.mil/sti/citations/ADA128940 (accessed on 22 June 2022).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sen, S.; Moazzen, E.; Acuna, S.; Draxler, E.; Segre, C.U.; Timofeeva, E.V. Nickel Hydroxide Nanofluid Cathodes with High Solid Loadings and Low Viscosity for Energy Storage Applications. Energies 2022, 15, 4728. https://doi.org/10.3390/en15134728
Sen S, Moazzen E, Acuna S, Draxler E, Segre CU, Timofeeva EV. Nickel Hydroxide Nanofluid Cathodes with High Solid Loadings and Low Viscosity for Energy Storage Applications. Energies. 2022; 15(13):4728. https://doi.org/10.3390/en15134728
Chicago/Turabian StyleSen, Sujat, Elahe Moazzen, Sinjin Acuna, Evan Draxler, Carlo U. Segre, and Elena V. Timofeeva. 2022. "Nickel Hydroxide Nanofluid Cathodes with High Solid Loadings and Low Viscosity for Energy Storage Applications" Energies 15, no. 13: 4728. https://doi.org/10.3390/en15134728