1. Introduction
Nanotechnology is contributing to industrial development in a wide range of areas, including information and communication, biotechnology, medical care, the environment, energy, and sensors. Dr. Eric Drexler of MIT in the United States popularized the term ‘nanotechnology’ in the late 1980s [
1]. He predicts that nanotechnology, capable of controlling materials on the nanometer scale, will change the world in the coming decades. In particular, it has been confirmed that the important concept in nanotechnology is not only size reductions but also the development of nanomaterials with unique shapes and structures. Nanotechnology has established a new technical field by connecting existing technical fields (e.g., physics, materials, and electronics). Nanomaterials are small, between 1 and 100 nm, and have excellent properties not observed in bulk forms. Size and shape control methods of nanoparticles are relatively simple. In addition, because they can be synthesized in an aqueous solution, there is an advantage in that a large number of materials can be synthesized relatively easily. Accordingly, various types of nanoparticles have been developed simultaneously [
2,
3]. The high ratio of the surface area to volume of nanoparticles increases the efficiency of catalysts and improves the sensitivity of sensors. In addition, particles synthesized with high purity levels have more advanced optical and electromagnetic properties, giving them many advantages in biological and clinical application research. As a result, the use of nano-biosensors has been expanded and now includes the detection of sensitive (or small amounts) cells in the human body and the ability to undertake precise examinations of local tissues in relation to medical diagnoses and clinical analyses. [
4,
5].
In general, particles used in high-sensitivity sensors include polymer nanoparticles, organic/carbon nanoparticles, biologically derived nanoparticles, and metal nanoparticles. Polymer-based nanoparticles are nano-sized solid particles that are synthesized by the polymerization of a monomer. Polymer nanoparticles may be less durable than metal nanoparticles, but they are widely used in medical fields because they can maintain strength levels similar to those of tissues and because they decompose in the body [
6,
7,
8]. Metal nanoparticles have excellent durability and have unique physical, chemical, and electrochemical properties according to their size. In addition, there is the advantage of being able to amplify signals generated by fluorescent materials and low-molecular labeling materials based on the material properties of the corresponding metals. For example, the plasmon resonance phenomenon of metal nanoparticles can have the effect of amplifying certain optical properties, such as the Raman signal and fluorescent molecular signal. Surface-modified metal particles have excellent potential if applied to the development of sensor functions such as the amplification of electrochemical signals and improvements of sensitivity and selectivity capabilities. Therefore, this technology has been widely used in recent research related to sensors [
9,
10,
11,
12]. Organic/carbon nanoparticles exist in various structures and forms, such as single-walled carbon nanotubes (SWNT), multi-walled carbon nanotubes (MWNT), graphene, and carbon quantum dots. If carbon-based nanoparticles are applied to the sensor field, the resulting nanoparticles can offer many advantages due to their high surface-area-to-volume ratio, excellent electrical conductivity, good chemical durability, and high mechanical strength [
13,
14,
15]. When a 3D material is nano-sized, it is referred to as a 0D material and is generally called a nanoparticle. A 1D material refers to a linear nanostructure having a lateral dimension of 100 nm or less. In addition, the 2D material has a layered structure similar to a thin sheet. 2D materials are characterized by a layered crystal structure with strong in-plane bonding, and the layers are bonded together by weak van der Waals (vdW) forces. Here, van der Waals force refers to the attraction between atoms, molecules, and surfaces. Van der Waals forces differ from covalent or ionic bonds because they are generated by polarization by nearby particles [
16,
17]. Unlike conventional quantum well semiconductors, control of the thicknesses of vdW semiconductors is done atomically. It is well known that device performance outcomes are degraded due to changes in the thickness of conventional semiconductor quantum wells [
18]. 2D materials can be used in various ways because they have unique optical and electronic mechanical properties, and the atomic thickness and exposed surface can be designed and controlled, broadening the application range. Furthermore, in recent years, synthetic research on the stacking of 2D materials is being conducted. Research is focusing on 2D-0D, 2D-1D, 2D-2D, and 2D-3D structures given their superior performance to 2D materials for application to a wider variety of fields [
19].
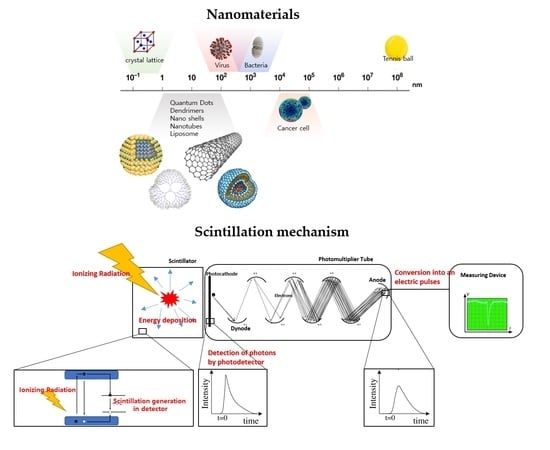
Recently, radiation detection sensors are being researched and developed for multiple sensors with small sizes, large areas, and good flexibility depending on the purpose. In particular, research on the development of sensors using nanomaterials as described above is being actively conducted worldwide. Research on and the development of radiation measurement and analysis technology has been steadily carried out in the nuclear field. And, as interest in radioactive waste and decommissioning has increased as an emerging issue. The development of radiation measurement sensors using nanomaterials has been carried out later than in other fields. When dismantling nuclear facilities, a large amount of radioactive waste is generated. Therefore, it is necessary to develop large measurement sensors because classification must be performed quickly after identifying the contamination level for radioactive waste. In addition, it is necessary to develop small sensors in multiple arrangements (e.g., Phoswich detectors) for radiation measurements and for continuous radiation monitoring in narrow places such as the pipes of nuclear facilities. With regard to decommissioning, high-purity semiconductor detectors (HPGe) or inorganic scintillators (NaI(Tl)) were widely used in the past, but these have disadvantages related to their size, complexity, and the need for expertise for these detectors. Plastic scintillators, which have many advantages in terms of utilization, show significantly lower detection performance capabilities than inorganic scintillators and semiconductor detectors. When a scintillator absorbs energy, electrons are excited and many electron-hole pairs are created. After a certain period (approximately 10
−7s), the electrons deexcite and light is emitted. At this time, the emitted light is in the visible energy region, and the energy incident on the scintillator is analyzed through the emitted light. When nanomaterials are added to this scintillation mechanism, the optical performance is improved. In the nano-volume, there exhibit unique optoelectronic properties due to the quantum confinement effect of electron-hole pairs. In other words, when nanomaterials interact with incident radiation, the photoluminescence increases because not only energy transfers but also momentum transfers between them are induced by the quantum mechanical movement of electrons [
9]. Using this principle, many studies have been conducted to develop functional nanomaterials with performance capabilities similar to those of semiconductor detectors or inorganic scintillators, with excellent process-ability and simplicity and that offer control of performance outcomes.
In this contribution, we review the present status of radiation detection and scintillator technology based on nanomaterials, including future prospects. In particular, the characteristics of organic as well as inorganic scintillators and nanomaterials are summarized and described in detail. In addition, functional materials for manufacturing high-performance scintillators and their fabrication methods are provided. Eventually, this review can serve as invaluable knowledge related to the development of nanomaterial-based scintillators.
5. Nanomaterial-Based Scintillators
In nanomaterials, the control of electron-hole pairs and the efficient generation of multiple excitons, that is, the generation of multiple electron-hole pairs by one photon, are unique photophysical properties of nanomaterials. In bulk scintillators (
Figure 10a), electron-hole pairs lose their energy while emitting phonons and are located at the edge of the band. On the other hand, in nanomaterials (
Figure 10b), the quantized energy level creates a phonon bottleneck, which can transfer energy to the creation of new electron-hole pairs instead of phonon emission [
91]. Therefore, unlike the bulk scintillator, the nanomaterial-based scintillator can improve the efficiency as well as control the emission wavelength according to the type and size of the nanomaterial used. In addition, there is an advantage that the reaction rate with photons can be improved by using nanomaterials with high atomic numbers. However, nanomaterials tend to aggregate because of their large specific surface area. Therefore, it is important to uniformly disperse the nanoparticles when depositing them on a polymer.
A reaction in which two or more molecules combine to form a compound with a high molecular weight is called polymerization. A thermal polymerization method is generally used when manufacturing a scintillator, and there is a polymerization method using light, radiation, or a catalyst.
Table 6 describes the nanomaterial doping and scintilaltor fabrication methods used in the research cases mentioned in this review.
The study of quantum dot synthesis methods was accelerated after the Bowendi group at MIT in the 1990s when they reported a quantum dot synthesis method of the high-quantum-efficiency cadmium series. Various quantum dots have been reported thus far, including the cadmium series, II–VI, III–V, IV–VI, and I–III–V. The properties of quantum dots also change depending on the material, and quantum dots, commonly called cores, do not have good luminous properties. However, there are reports of ways to maximize these characteristics by wrapping them with a shell structure or creating them in an alloy form. These methods can simultaneously increase the efficiency and stability of quantum dots.
In 2018, Chen Q. et al. [
92] conducted a study using perovskite nanocrystals. In a typical bulk scintillator material, it interacts with high-atomic-number materials to generate many photoelectrons through photoelectric effects. It is possible to control the color of perovskite quantum dots by controlling the emission wavelength, resulting in a narrow half-peak emission peak. However, the emission spectrum of the bulk scintillator is uncontrollable and shows an emission peak with a large half width. In that study, these properties of perovskite ionization crystals were utilized to develop a prototype device for multi-colored X-ray detection through a combination of solution processing and soft lithography, with a subsequent comparison with commercial bulk scintillators (CsI:Tl, PbWO
4, YAlO
3:Ce and Bi
4Ge
3O
12). After analyzing the X-ray absorption spectrum of carbon, CdTe, and CsPbBr3, the authors of the paper [
92] found that Pb-based perovskite nanocrystals are more suitable for photon detection scintillators than nanocrystals without Pb components. In addition, the authors observed that the bandgap energy of perovskite nanocrystals is in the range of 1.7~3 eV and that the energy density reaches its maximum when the atomic distance is 10.32 Å. The prototype device developed in the study was manufactured by spin-coating a perovskite material, and it was confirmed that the lowest detection dose rate was 13 nGy/s. This value is approximately 420 times lower than the dose (5.5 uGy/s) used for a general X-ray diagnosis. In addition, it was confirmed that a very rapid response (τ = 44.6 ns) was obtained from the Cs-137 source. Rapid responses to X-rays are crucial important factors in medical radiography. In addition, the characteristics of the perovskite and commercial bulk scintillators (CsI; Tl, PbWO
4, YAlO
3; Ce and Bi
4Ge
3O
12) were compared. The perovskite showed good luminescence sensitivity to X-rays, with multi-color high-efficiency luminescence found to be possible. On the other hand, conventional scintillators have poor resolutions of the emission peaks. Therefore, it is difficult to express multi-color visualization by photons. In the aforementioned study, it was confirmed that perovskite nanocrystals showed a very rapid response (44.6 ns) to X-rays, and the scintillator using the perovskite can be converted into multi-colored visible light, which has a small half width. It was also confirmed that use as a scintillator was possible. The results of the study appear to be applicable to the X-ray sensing and the imaging industry, with perovskite materials also useful as alternative materials in solar and light-emitting diode applications.
In 2019, Chang S. et al. [
93] conducted a study to improve the luminescence using quantum dots and developed a method of uniformly doping quantum dots covered a thiol. Here, the thiol is an organic sulfur compound (R-SH) structure, R is an alkyl or other organic substituent, and -SH denotes a sulfhydryl group (sulfhydryl group). The thiol (R-SH) binds to the quantum dot and protects the surface of the quantum dot. Samples were prepared using three types of quantum dots (MPA-CdTe, MPA-CdSe, and Cys-CdTe) and the properties were evaluated. MPA is mercaptopropionic acid, and Cys stands for cysteamine. As a result of evaluating the characteristics of quantum dots using the emission spectra, TEM, and XRD patterns, it was confirmed that the type and particle sizes of quantum dots influence their luminescence and quantum efficiency. These results are confirmed in
Figure 11 and
Figure 12 in that study, three types of quantum dots were synthesized, and the synthesized quantum dots were enhanced by a chemical reaction with photons by a high-energy gamma irradiation method in an aqueous solution. Photons can interact with water molecules, organic ligands (MPA or Cys) and inorganic nanocrystals (CdTe or CdSe). Water molecules are decomposed by photons to generate active species such as H
−, HO
−, HO
2−, and they interact with nanocrystals such as CdTe or CdSe to release Cd
2+ ions. S
2− and Cd
2+ can also be generated by direct interaction between photons and quantum dots. The resulting S
2− and Cd
2+ combine rapidly to form CdS, with the photons releasing S
2− and Cd
2+ very slowly such that a CdS shell is formed on the quantum dot surface rather than the on CdS particles in the solution. Thus, a thin CdS shell can reduce defects on the CdTe or CdSe quantum dot surfaces. Because the bandgap of CdS is wider than those of CdTe and CdSe, the formation of a thin CdS shell is considered a key factor in improving the fluorescence efficiency of quantum dots. The gamma irradiation method used in this study has the following advantages: (1) Gamma rays have high energy and improve the fluorescence of quantum dots in a short period of time. (2) Gamma rays have strong penetrating power and uniformly react chemically in the entire system, leading to the homogeneity of substances. (3) Gamma rays can uniformly process large amounts of quantum dots at a time. Compared to the conventional chemical method, the gamma emission method is generally performed at atmospheric pressure and room temperature in an aqueous solution without a large amount of chemicals, which has the advantage of reducing damage to other substances and reducing environmental contamination. Therefore, using this photon irradiation method, it is possible to process large amounts of quantum dots uniformly at one time [
93].
In 2009, Joe M.J. et al. [
94] succeeded in synthesizing a new type of microparticle by uniformly doping quantum dots on the surface of a silica sphere and then growing a silica layer on the quantum dots.
Figure 13a shows a multi-size CdSe quantum dot synthesized from the Korea University study. The smaller the quantum dot size is, the shorter the emission wavelength is. The synthesized CdSe quantum dots were measured with UV-Vis spectroscopy; the band edge emission (BEE) was found to be 566 nm, with the BEE also measured and found to be 575 nm. This was calculated as 3.4 nm using the formula below to calculate the size of the quantum dots. In addition, after composing a 2.5-layer CdS shell on the surface of CdSe, the UV-Vis spectroscopy measurement showed that the band edge absorption (BEA) was 594 nm (28 nm movement after shell formation) and the BEE was 603 nm (28 nm movement after shell formation).
Figure 13b shows the UV absorption and fluorescence spectra of CdSe quantum dots (
Figure 13b(1)) and CdSe/CdS quantum dots (
Figure 13b(2)). When the size of the CdSe/CdS quantum dot is calculated based on
Figure 13b and Equation (1), the outcome is 4.33 nm.
Here, λ means the wavelength at the BEA position.
Thus, the study by Joe M.J. et al. demonstrated the uniform formation of a layer of quantum dot particles on the surface of the silica sphere to minimize self-absorption and agglomeration without using an organic polymer. Additionally, the silica layer was grown to synthesize particles with amplified fluorescence, preserved fluorescent wavelengths, and increased light stability and durability.
In 2006, Letant S.E. et al. [
95] developed a QD composite material of porous materials with improved resolutions compared to that of the NaI(Tl) scintillator, an inorganic scintillator. Inorganic scintillators such as NaI and CsI have poor resolutions, and CZT has a resolution of about 1% at 662 keV, but it cannot be manufactured in a large size. For this reason, research has concentrated on the development of gamma measurement scintillators using nanomaterials.
In this study, nanocomposite materials were developed using porous VYCOR and CdSe/ZnS quantum dot materials. The manufacturing method utilized in this case is as follows. A 1/16-inch porous VYCOR (high silicate glass (96% silica glass)) sheet was slowly dissolved in a solution containing 1% hydrofluoric acid and 2.0% ethanol per volume for four days. The solution was prepared by mixing CdSe/ZnS quantum dots having an emission wavelength of 510 nm for 48 h and then slowly cooling them (air-dried).
Figure 14 shows the nanocomposite material added with the porous VYCOR-based CdSe/ZnS quantum dot material produced in the study. To understand the performance of the produced nanocomposite material, a 25-mm-thick nanocomposite material was connected to a PMT (model R1924A) to perform measurement experiments, and the results were compared with those from a one-inch NaI(Tl) scintillator.
Figure 15 shows the results of a measurement experiment using the Americium-241 source. The nanocomposite showed a resolution of 15% in the Americium-241 (59 keV) region, and the NaI(Tl) scintillator showed a 30% resolution in the Americium-241 (59 keV) region. The nanocomposite material developed in this study thus showed a resolution nearly two times better in the Americium-241 (59 keV) region. Although this study proved that a semiconductor detector using quantum dots has adequate photon output at a low gamma energy level, scintillation and linearity studies to assess the high gamma energy must be performed. In order to generate high detection efficiency, a high-density thick nanocomposite material must be devised to increase the stopping ability, and the Stokes shift of the quantum dots should be maximized to prevent losses due to reabsorption in the thick detector structure [
95].
In 2012, Lawrence W.G. et al. [
96] used a polymer composite thin film doped with quantum dots for X-ray imaging. Quantum dots/polymer thin film samples were prepared using both aqueous CdTe quantum dots of polyvinyl alcohol and non-aqueous CdSe quantum dots of polystyrene. The surface of the quantum dot was stabilized in an aqueous solution and coated with an appropriate ligand to prevent aggregation. A CdTe quantum dot thin film sample consisting of a polyvinyl alcohol (PVA) polymer was prepared in an aqueous solution containing water-soluble quantum dots and polymers, with samples 65 μm thick created in a 5-mm-thick optical fiber optical guide plate with 25 mm diameter. Polystyrene samples doped with CdSe quantum dots were prepared by mixing styrene and quantum dots and then thermally polymerizing the mixture using AlBN (2,2’-azobisisobutyronitrile). They were prepared as a solid sample 10 mm in diameter and 5 mm thick. In addition, the prepared samples were polished for light transmission of the upper and lower surfaces. In this study, the characterization of the polymer with the addition of quantum dots was evaluated only for samples with the addition of CdTe quantum dots. As a result of the evaluation, the samples to which 10% quantum dots were added were uniformly mixed, but the agglomeration phenomenon was observed in the samples containing 35% quantum dots. An analysis showed that the lower the content of the quantum dot was, the better the uniformity on the film surface became. However, as the quantum dot content increased, the luminous efficiency increased [
96]. Therefore, it is considered that additional research on a method capable of uniform doping at a high content is necessary.
In 2017, Liu C. et al. [
97] conducted a study to develop a nanocomposite material with improved light yield and better measurement efficiency over conventional materials for gamma detection. The ideal scintillator has the following characteristics. First has a high atomic number that increases gamma attenuation and the photoelectron generation rate. Second, it has a high light yield (visible light generated per incident photon energy (MeV)). The third characteristic is the short decay time, and the fourth is its low cost such that it can be manufactured at a large size. However, commercial scintillators are generally made of inorganic single crystals or plastics and do not match the characteristics of an ideal scintillator due to limitations in the manufacturing process and the inherent characteristics of the materials. The manufacturing method of the plastic scintillator with quantum dots added to it is as follows. Separated from a toluene solution was done using ethanol to obtain 1000 mg of quantum dots. The separated quantum dots were dissolved in 14 mL of CHCl
3 to form a transparent solution, and 89 mg of BMEP(bis(2-(methacryloyloxy)-ethyl) phosphate) in 2 mL of CHCl
3 was stirred into the solution. The mixture was stirred for 24 h and then filtered through a 200 nm PTFE filter to remove the precipitate which formed upon stirring. The filtered solution was washed twice with a solution of 3:1 acetone and toluene and then twice more with a solution of 3:1 acetone and hexane. Subsequently, the transparent solution was placed in a glove box filled with nitrogen and mixed with 1–5 wt% of FBtF(4,7-bis-{2′-9′,9′-bis[(2″-ethylhexyl)-fluorenyl]}-2,1,3-benzothiadiazole) in a glass vial with a diameter of 10 mm, followed by thermal curing at 95 °C for 24 h. The resulting monolith was separated from the glass vial and polished to produce a quantum-dot-based polyvinyl toluene (QD/PVT) scintillator. In this study, by adding more than 60 wt% of CdZnS/ZnS (CZS) nanomaterials to the existing plastic material, nanocomposite materials with a 9.8% resolution in a 662 keV Cs-137 gamma peak were successfully produced with a resolution similar to those of inorganic scintillators such as BGO, CaF
2(Eu) and CsI(Tl), meaning that it is a material with performance capabilities similar to those of inorganic scintillators at an affordable price. To increase the atomic number of the nanocomposite material and improve the light yield, it was prepared by adding more than 60 wt% of CZS QD to the PVT matrix with a sufficient amount of FBtF dye. When a high amount of quantum dot material is added, the distance between the quantum dots is close, leading to the FRET phenomenon. The FRET phenomenon refers to a phenomenon in which energy does not emit light at the absorbed wavelength and the wavelength shifts to emit energy at other wavelengths. Therefore, by utilizing the FRET of FBtF QDs having a low bandgap, QD self-absorption is prevented, energy is emitted at a shifted wavelength, and the light yield is improved by 11% at 662 keV compared to an existing nanocomposite scintillator. The material content showing the best performance was the 60 wt% CZS QD and 2% FBtF/PVT nanocomposite scintillator, the light yield was 9255 photons/MeV, and the resolution was 9.8% at 662 keV. This is shown in
Figure 16 [
97].
In 2018, Tam A.K. et al. [
98] developed a nano-scintillation material by adding CdS quantum dots to a base of PVT to increase the luminescence rate of application to a scintillator. A PPO material was used as a wavelength shifter together with a quantum dot material. PPO and CdS quantum dots having an emission wavelength of 418 nm coated with oleic acid were dispersed in a PVT matrix to prepare the PVT with the quantum dots added. As a result of evaluating the performance of the PVT produced in this study, energy transfer was observed in three stages from the PVT polymer to PPO and from the PPO to CdS quantum dots under UV excitation. Beta and gamma spectroscopy were conducted with various sources to evaluate the performance of the quantum dot-based scintillator. Gamma interaction with the scintillator is likely to lead to interactions such as a photoelectric effect and the Compton effect due to the low atomic number of the polymer. It is assumed that the majority of the light yield is due to the interaction with the beta particles, which have a very high stopping ability for the polymer. Gamma and beta measurements were conducted for each sample for 20 min through a scintillation experiment on a polymer sample to which various (0–0.2 wt%) concentrations of quantum dots were added using an unshielded Cs-137 source. The total cps (count per second) of each sample is shown in
Table 7, and the spectrum is shown in
Figure 17. It was confirmed from
Table 7 that the total cps approximately tripled as the amount of quantum dots was increased to 0–5 mg. Through this, it could be demonstrated that the addition of quantum dots improves scintillation. In addition, the CdS quantum dots were proved to be a substance that can be added to improve scintillation through this study [
98].
In 2011, Park J.M. [
99] created a plastic scintillator with quantum dots added to it and conducted a study to check the wavelength shift.
Although the plastic scintillator is not always used for gamma-ray measurements, in this study, a QD-based plastic scintillator with improved performance. An experiment was conducted to assess the luminescence characteristics at different quantum dot concentrations. In this case, quantum dots were used to realize a wavelength shift. A plastic scintillator doped with quantum dots was manufactured by a thermal polymerization method, and the luminescence properties were determined by X-rays. In addition, the decay time was confirmed through gamma-ray spectroscopy. At this time, the photodetector used in the gamma-ray spectroscopy analysis utilized PMT (PHOTONIS XP2260/PA). The PMT emission wavelength of the PHOTONIS device is 400 nm, and the silicon optical sensor shows a wavelength of 600 nm. In general, the wavelength band of a plastic scintillator is around 400 nm, meaning that PMT, not a silicon optical sensor, was used.
Table 8 shows the materials and concentrations used in the production of the plastic scintillator. CdTe/ZnS was used as the quantum dots in this experiment, and the quantum dots were also a material produced through a synthesis process [
99].
In 2014, Park J.M. et al. [
100] assessed the properties of scintillators after adding CdSe/ZnS QDs to plastic-based materials. A plastic scintillator containing quantum dots was created via a thermal polymerization method as described above and was prepared with the contents shown in
Table 8. The manufacturing method used to create the plastic scintillator is identical to that shown in
Figure 18. The entire process was performed in glass bottles with a diameter of approximately 2.5 cm and a thickness of 5 cm. The produced scintillator was irradiated with a 10 nA 45 MeV proton beam for ten seconds to obtain the proton-derived spectrum.
Figure 19 shows the proton spectra of the #4 and #5 samples, showing the same emission peak at 350 nm and 520 nm. However, the emission intensity of each peak is different. The light yield of the #5 scintillator was 3.5 times stronger than that of the #4 scintillator at 380 nm, and the light yield of the #4 scintillator was 1.3 times stronger than that of the #5 scintillator at 520 nm. It was also found that larger quantum dot contents led to a higher 520 nm peak was due to the higher energy transfer from the PPO to the quantum dots. Because the emission wavelength must match the photodetector, the wavelength band can be controlled using quantum dots. In this study, the wavelength shift was checked for each plastic scintillator made of quantum dots of various concentrations, and the decay time was checked using a gamma-ray source. The decay times of standard plastic scintillators (Styrene, PPO, POPOP) are 4.4 ns and 16 ns, and those of corresponding QD (0.05 wt%) doped plastic scintillators were measured and found to be 2.40 ns and 11.4 ns. The results of this study are considered to be applicable to the effort to establish a method for the manufacturing of plastic scintillators including quantum dots [
99,
100].
In 2019, Brus V.V. et al. [
101] developed a large-area X-ray and γ-ray detector with a Schottky double junction of graphene / CdTe crystals. A device using two materials was prepared by forming a van der Waals contact between graphene and a CdTe substrate by a large-area chemical vapor deposition method at room temperature. This method greatly reduces the manufacturing costs and improves the reproducibility and stability of the electrical properties. The thin graphene layer does not interfere with the low-energy X-rays to the active layer, and the relatively high sheet resistance of the single-layer graphene is not a problem because, as opposed to a solar cell, the electrical signal generated by the radiation detector or photodiode is low. The operating range of the CdTe detector is higher than that of the Si detector, giving it a higher atomic number and enabling measurements of higher photon energy levels with a corresponding wider bandgap. However, several problems related to the physical properties of Schottky diodes based on high-resistance materials were not solved in this study. High-resistance CdTe single crystals are associated with a significant temperature instability issue and the electrical properties cannot be controlled. In this study, a radiation detector was developed using the new graphene/CdTe single crystal double junction method, excluding a vacuum deposition process, and the spectral characteristics were confirmed with the developed detector. The graphene/CdTe/Au detector and the circuit diagram developed in this study are shown in
Figure 20. To verify the performance of the developed Schottky diode-type semiconductor detector, the spectrum of Am-241 and Cs-137 was analyzed. These results are shown in
Figure 21. As a result of these measurements, the resolution was found to be 21% in the Am-241 (59 keV) region and 6.1% in the Cs-137 (662 keV) region. This showed better performance than NaI (Tl) with its resolution of about 7–8% in the Cs-137 region [
101].
In 2019, Kim S.J. et al. [
102] developed a gamma-ray detector based on a single-wall carbon nanotube (SWCNT). SWCNT can be described as not having an existing scintillation mechanism. When gamma rays are incident, two SWCNT resistors sense and change the electrical properties due to oxygen dissociation and adsorption on the SWCNT surface. With the developed detector, the total dose and dose rate showed excellent sensitivity, and the radiation dose rate was distinguished in the range of 2.4 to 16.4 R/min. In this study, a simple and economical method for gamma-ray detection using SWCNT without other scintillators was established. The SWCNT network was devised with two terminal resistors and the resistance was measured in real-time with various gamma doses and various dose rates. During these measurements, ambient air is exposed to the environment due to adsorption of oxygen atoms and ozone, and a control sample was prepared with a nanotube surface coated with a polymer to prevent a reaction with the environment in a simultaneous investigation.
Figure 22 shows a schematic diagram of the manufacturing process of the gamma-ray detector. In this experiment, this device was prepared using an ink-based drop-casting method [
102].
The SWCNT ink has a semiconductor nanotube content of 1 wt% in a diameter range of 1 to 1.3 nm and contains 80% or more in a solvent. The impurities in SWCNT are mainly metallic nanotubes, not other contaminants. In addition, the SWCNT ink was further diluted with deionized water to generate various nanotubes, and the nanotube concentration was optimized to maximize the radiation response.
High-energy gamma rays are generated when oxygen atoms dissociate when colliding with O
2, and oxygen atoms combine with oxygen molecules to form ozone molecules. Ozone production by gamma rays has been known for a long time, and ozone generation rates have been reported at various distances from the gamma source. For example, according to a previous study, the amount of ozone generated in a 20 L flask filled with air was measured and found to be approximately 7.78 to 13.45 ppm for a total capacity of 850–1127 krad; another study showed values of 8 ppm for 50 krad and 58 ppm for 1000 krad. However, as opposed to desorption by UV, oxygen adsorption with these nanotubes leads to a decrease in the resistance.
Figure 23 shows the air-coated, PET-coated, and PDMS-coated detectors. In two of the coated samples, SWCNT was coated with a polymer film to separate the active area from the ambient air while completely exposing the active area to air. The thin PET film was laminated to cover the nanotubes without tight contact and without confining the limited amount of air in the cover. When the sample was irradiated at an exposure dose rate of 16.41 R/min (14.4 Rad/min), as shown in
Figure 24, the polymer coating detector showed a tendency to have no resistance change, and the resistance of the detector exposed to air showed a tendency to decrease. In the PET sample, the trapped air is insufficient to cause ozone-induced resistance movement. According to these results, adsorption by oxygen products is an important sensing mechanism. When oxygen is contained in the sample, it is mainly attached to the defective portion of the nanotube. Theoretical studies showed strong interactions between the nanotubes and ozone as long as there is ozone adjacent to the nanotubes as produced by the interaction between gamma rays and air.
In this study, an SWCNT-based gamma-ray detector was fabricated to demonstrate its performance under various exposure conditions. The detector distinguished between sensitivity to gamma rays and radiation dose rates in the irradiated range. The adsorption of oxygen, especially ozone molecules, appears to influence the CNT response to radiation, and direct radiation damage to nanotubes has been found not to play a role in this. This study provided basic data for the development of a new gamma-ray detector using nanomaterials based on an indirect sensing mechanism [
102].
In 2016, Kang C.G. et al. [
103] conducted research on the development of a flexible radiation detector. There are several types of existing radiation detectors, but the scintillator type of radiation detector has a structure in which a scintillator that generates light when radiation is incident and a photodetector that detects the generated light is combined. The scintillator of the existing photodetector is bulky and sturdy and has a high manufacturing cost. Given its hard silicon semiconductor wafer as the base, it cannot bend, meaning that there is a limit to the shape of the attached surface of the scintillator. Therefore, research on the development of flexible photodetectors using nanomaterials as photodetectors was conducted. The advantage of this technology is that it offers manufacturing in various shapes given the thin and flexible material without limits in terms of the measurement location. In addition, because it can be produced through a printing process, there is the advantage of a low manufacturing cost.
Figure 25 shows the electron transfer with this patented technology. In
Figure 25, ① is the scintillator part, and photoelectrons or Compton electrons are emitted by the interaction between gamma rays and the scintillator (photoelectric effect, Compton effect) to excite the scintillator (e.g., NaI). After being excited as a conduction band, visible light is emitted while transitioning again(within 10
−8 sec). In addition, ② is the insulator part, which serves to block the direct transmission of the electrical signal generated by the scintillator to the photodetector. It is formed to a thickness of about 30 to 50 nm and is composed of oxides such as SiO
2 and HfO
2 or nitrides such as SiNx and AlNx. ③ is the active layer (photoelectrode), and it emits photoelectrons by interacting with the light generated from the scintillator (photoelectric effect); the active layer can be composed of a substance with the formula of graphene, graphene oxide, graphene quantum dots, and MX2. In order to increase the light transmittance, it can be manufactured by repeatedly stacking nanomaterials. The quantum efficiency represents the sensitivity of the photocathode and is usually about 20~30%. The nanomaterial of the active layer is patterned (the action of etching a desired circuit or shape on a substrate) by inkjet printing process technology. Because its form is thin once patterned, it is patterned several times to prevent permeability. ④ is the electrode that collects the generated electrons and converts them into electrical pulses. It can be made of copper, gold, platinum, palladium, and other similar metals with good electrical conductivity. The photodetector is manufactured as shown in
Figure 26. After forming a photodetector on a silicon oxide substrate, a temporary substrate is formed for photodetection. Thereafter, the silicon oxide substrate is chemically removed by precipitation in a SiO
2 etching solution, and a temporary substrate with a photodetector is attached to the scintillator. The temporary substrate is removed through a chemical reaction by precipitating it in an acetone solution after a drying process. Using this patented technology, it is possible to attach a photodetector to a scintillator of various shapes, and it is expected that the shape of the scintillator attachment surface will not be restricted.
Kang D.H. et al. [
104] implemented a high-performance photodetector with a light response of 1.27 and 6A/W by utilizing doping and lamination technology of two-dimensional nano-semiconductors. Here, doping technology refers to a process of changing the characteristics of a semiconductor by adjusting the concentration of electrons or holes, and photo-responsiveness refers to the amount of current generated by electrons and holes generated per unit intensity of incident light. Optical characterization is the most representative method to know the performance of a detector. Two-dimensional nano-semiconductors generally have only
n-type or
p-type properties, depending on the type of material used. The
n-type refers to a semiconductor in which electrons are the main carriers that dominate the electrical conductivity of semiconductors, and, conversely, when holes are the main carriers, it is the
p-type semiconductor. Owing to these characteristics, a study was conducted to improve the performance of application devices such as photodetectors and transistors by variously controlling the operating characteristics of the semiconductors through doping. The research team at Sungkyunkwan University reversed this by applying n-type doping technology to supply electrons by thinly coating triphenylphosphine (PPh
3) onto the surface of tungsten selenide (WSe
2), a two-dimensional nano-semiconductor material with p-type operating characteristics. They implemented an
n-type photodetector and confirmed that the photo responsiveness is one step higher than that of an existing
p-type WSe
2 photodetector. In addition, a two-dimensional insulating material, hexagonal boron nitride (h-BN, chemical and physical properties similar to graphite, but graphite is an electrically conductive material, while h-BN is an excellent electrical insulator), is a vertical stack of two-dimensional nano-semiconductors. By utilizing a layered structure, the photo-response is improved via the creation of a high-quality interface with a low defect density between the hetero-materials. Compared with results from an existing molybdenum disulfide (MoS
2)-based photodetector device, the photo-response is increased by about 25 times, and the result with the high-performance photodetector at 6A/W is 1.27, meaning that it can detect light of very weak intensity. In addition, compared to the photo-response of 5A/W of the
p-type tungsten selenide (WSe
2) photodetector to which doping and lamination technologies are not applied, the result is improved by nearly five times, showing the superiority of this technology. This is more than one million (10
6) times higher than photodetectors based on silicon (Si) and gallium arsenide (GaAs), which are widely used semiconductor materials [
104]. Because the technology developed in this study can be widely used in various 2D electronic/photoelectric device processes, it is considered that it can be used with nanomaterial-based plastic scintillators in the future.
In 2015, Shin D.H. et al. [
105] conducted research on the development of graphene quantum dot optical sensors with improved photo-reactivity. Currently, silicon (Si) or indium gallium arsenide (InGaAs) is used as the material of the photodetector, but this material is limited when used in a transparent optoelectronic device that can be folded because it is hard and opaque. In order to solve this problem, researchers attempted to utilize two-dimensional materials such as graphene and graphene quantum dots for transparent and flexible optoelectronic devices. As a method to improve the device performance of graphene quantum dots, nanoparticles that generate a large number of electron-hole pairs by high light absorption were made to be adjacent to the graphene quantum dots, and the nanoparticles in electron-hole pairs were generated from the graphene quantum dots. This was intended to increase the efficiency of the device by adding electron-hole pairs generated by light absorption. In Shin’s research team, as a first application of a device using a graphene quantum dot-nanoparticle energy transfer structure, a graphene quantum-dot nanoparticle photodetector (optical sensor) using graphene as a transparent electrode was fabricated and its characteristics were evaluated. The developed photodetector has an energy conversion layer with a hybrid structure in which graphene quantum dots (GQDs) are bonded to the surface of silica nanoparticles (SNPs), with a second electrode formed by transferring graphene onto the energy conversion layer also formed. In the energy conversion layer, silica nanoparticles function as energy donors and graphene quantum dots act as energy acceptors; the charge carriers generated on the surfaces of the silica nanoparticles due to light absorption are transferred to graphene quantum dots in an energy exchange mechanism that involves the reception and transmittance of energy. The developed photodetector showed increased photo reactivity of more than 1000 times compared to a photodetector with only graphene quantum dots. This is more than 100 times higher than the commonly used photodetectors based on silicon and gallium arsenide [
105].
In 2019, Heo J.H. et al. [
106] conducted research on the development of X-ray imaging technology using a perovskite nano-scintillator. X-ray detectors have received much attention given their numerous potential applications, such as in crystallography and space exploration and for medical diagnosis and safety inspections. Thallium-doped cesium iodide (CsI;Tl) and terbium-doped gadolinium oxide (GSO) are used as the scintillators of indirect X-ray detectors. The CsI;Tl scintillator has the advantage of a high resolution due to the vertically oriented column structure but has a disadvantage in that it is produced through a vacuum process with a long process time and is expensive. On the other hand, the GSO scintillator is composed of micro-sized GSO particles and a polymer binder, offering the advantage of low flexibility and a low cost, but one disadvantage is that the resolution of these devices is reduced due to light scattering. In particular, X-ray detectors for medical diagnosis, such as mammography, chest X-rays, tomography, and dental oral X-rays, have high sensitivity, high resolutions, high durability, and high-speed response characteristics to minimize radiation exposure to patients and obtain high-resolution images. Recently, Korea University developed a new X-ray scintillator that is superior to the existing X-ray scintillators by using CsPbBr3 perovskite nanocrystals, which are new luminescent materials for use in next-generation light-emitting diodes. Perovskite is actively researched for application as a next-generation solar cell or light-emitting diode (LED) material, and perovskite nano-scintillators that absorb X-rays have high luminous efficiency and excellent spatial resolutions. Because the instantaneous light emission time is very short, it is possible to reduce radiation exposure when obtaining an X-ray image. However, perovskite light-emitting diodes are relatively inferior in terms of stability. Therefore, further research is needed to meet commercial demands [
106].
In 2019, Kim G.W. et al. [
107] conducted research on the development of next-generation transistors for new technologies of ultra-fine semiconductor (graphene quantum dots). Graphene is a layer of atoms in which carbon atoms are connected in a hexagonal honeycomb shape. It is a new material that is thin, has high physicochemical safety, and has excellent electrical conductivity. If foreign matter can be formed at small dimensions of several nanometers, it can become a graphene quantum dot of ultrafine semiconductor particles. In the lab of the UNIST Department of Natural Sciences, in 2019 graphene quantum dots were developed more effectively than ever before. It is believed that this technology will contribute to the development of “single-electron transistors”. Transistors are components of semiconductor-integrated circuits that amplify or alter electronic signals. Currently, electron flows of 100,000 electrons are controlled to operate one transistor, but single-electron transistors control only one electron, meaning that there is little power consumption and heat generation, and research is actively being conducted for future electronic devices. UNIST has developed a technology for manufacturing a ‘two-dimensional planar complex in which graphene quantum dots are regularly arranged in a single layer of hexagonal boron nitride (h-BN)’. With this material, we implemented a “vertical tunneling single-electron transistor”, a device that transmits a signal by controlling only one electron. Hexagonal boron nitride is a layer of atoms in which nitrogen and boron are combined in a hexagonal honeycomb shape, also called ‘white graphene’. Unlike graphene, it has an insulating property that does not allow a flow of current and can thus be applied as a two-dimensional non-conductor. In addition, one characteristic of graphene quantum dots is that they can emit light when current is passed through or light is radiated. For this reason, they have been spotlighted as a material for next-generation displays, bio-imaging, and sensors. They can also be applied to next-generation quantum information and communication technologies that can process information quickly while using less electricity. Thus far, graphene quantum dots have been made by chemical peeling, a technique of thinly peeling a graphite mass by a physical method or a chemical reaction. With these methods, it is difficult to obtain a graphene quantum dot of a desired size, and various impurities are attached to the edges, thereby blocking the flow of electrons. As a result, graphene quantum dots do not readily exhibit natural electrical and optical properties. The process of manufacturing a new 2D planar composite in which graphene quantum dots are formed in a hexagonal boron nitride monolayer in this study is shown in
Figure 27. First, the block copolymer (block copolymer) and H
2 PtCl
6 (the initial material for forming platinum nanoparticles) are mixed and applied onto a silica substrate, followed by a heat treatment. Graphene quantum dots are prepared by selectively substituting boron nitride to replace graphene onto the nanoparticles with graphene. In this technology, a new method was devised to control the size of the graphene quantum dots as desired and to remove impurities at the edges. Hexagonal boron nitride was transferred onto a silica (SiO
2) substrate with platinum nanoparticles and heat-treated in methane gas. The platinum nanoparticles are arranged regularly due to the self-assembling properties of the block copolymer, and the hexagonal boron nitride on the platinum swaps with graphene. As a result, the size of the graphene quantum dots is determined according to the size of the platinum particles, and a two-dimensional planar complex structure in which graphene quantum dots are regularly arranged in the hexagonal boron nitride layer of the atomic layer is created [
107].
Many studies have been conducted to improve the performance by optimizing the types of nanomaterials used or modifying the nanomaterials through manufacturing and doping methods. The research cases presented above are summarized in
Table 9.
6. Conclusions and Prospects
In this contribution, the concepts for synthesis, fabrication methods of nanomaterials as well as the present status of the development of scintillators based on nanomaterials are extensively summarized. Recently, many studies are being reported with the goal of determining detection limits at a low level quickly and accurately, and more recently in-situ methods are used to take into account economic feasibility and efficiency, including sample measurements. Although there is the advantage of the direct measurement of radiation at the site, saving time and money, and not producing secondary radioactive waste, it is inevitable to study the development of large-size detectors for quick and accurate measurements. Plastic detectors are made up of low-atomic-number materials and are widely used for beta-ray measurements because of their low-density levels. They can also be produced in various forms and at large sizes, making them popular for use in radioactive monitoring systems and whole-body counters. However, due to the nature of radiological detectors, it is not possible to analyze the nuclear species. Accordingly, inorganic scintillator detectors or semiconductor detectors are used for precise radiological testing. Inorganic scintillators have relatively good scintillation efficiency rates and resolutions and are therefore used for gamma-ray spectra, but they are difficult and expensive to manufacture at a large size. Therefore, if a plastic scintillator with performance capabilities similar to those of an inorganic scintillator is developed, it can be used for measurements at dismantling sites or large-capacity deconstructed waste sites, and it can also be used in the field of nuclear medicine. Recent studies have confirmed that adding nanomaterials to plastic-based materials affects the energy bands, resulting in new electrical, optical and magnetic properties. Therefore, if nanomaterials are used, it is expected that high-performance plastic detectors can be developed by increasing the luminescence efficiency.
Many studies are also being carried out in various fields using quantum dots, where the light-emitting wavelengths change with the size of the nanomaterials used. Although research activities and commercial use have increased at present, nanomaterials are associated with high costs. Additionally, there is the problem of the lack of mass production technologies for nanomaterials. The conventional synthesis method limits the amount that can be produced in a single reaction, placing many restrictions on the supply of uniform samples that are expensive and guarantee a certain property. For this reason, there is the problem of not using a large amount of materials, and when a large amount of a material is needed, it is necessary to make and use it for the specific purpose. An analysis of domestic and foreign synthetic technologies shows that mass production methods of quantum dots have yet to be resolved. Research to solve this problem is, however, being actively carried out. For quantum dots, the luminescence is known to vary sensitively depending on the condition of the surface, which acts as a limiting factor affecting the applicability of CdSe quantum dots synthesized in organic solvents. The development of technology to distribute hydrophobic CdSe or its derivatives to a variety of solvents that are polar or tragic without reducing the luminance is expected to enable the manufacturing of various hybrid compounds, including semiconductor quantum dots, while also increasing applications to more areas. Thus far, most quantum dots with outstanding optical properties are cadmium-based quantum dots, perovskites, and graphene. Cadmium-based quantum dots present a major obstacle to the commercialization of quantum dots, as they are also not environmentally friendly and only small quantities that are difficult to industrialize can be produced, they also have toxicity problems. Currently, InP and many other types of quantum dots of the III-V type are being studied and methods have been developed to maximize the characteristics of the quantum dots examined earlier, resulting in many short-term results, but optical characteristics are still lacking compared to those of cadmium-based quantum dots. Therefore, it is necessary to supplement the problems associated with toxicity and the environment as well as the problem of the mass production of high-quality quantum dots or to study new quantum dots that can solve these problems. Quantum dots are now being used considerably in the display and solar cell markets, and quantum dots using new materials are also being steadily researched. Thus, quantum dots are highly efficient materials in many areas beyond the present level of efficiency. 2D materials are a source technology applicable to various industrial fields, such as the Internet of Things (IoT), bending devices, ultra-low-power devices, next-generation batteries, water purification filters, and spacecraft, and research on high-quality, large-scale production and applications of materials at basic stages is being actively carried out to commercialize 2D materials in various industries. Although the large-scale production of a number of 2D materials has been successful, these efforts have not reached the quality of semiconductor wafers currently in service, and research is being conducted to improve them. Nanomaterials are active in industries such as semiconductors and displays and thus there is a certain demand market, and because there are no products still being mass-produced, it is believed that it will be possible to realize the industrialization of nanomaterials if large-scale high-quality nanomaterial mass-production technology is secured by intensive investments. In addition, large-size and high-efficiency scintillators that utilize nanomaterials can be developed not only in the semiconductor and display fields but also in the radiation/functional measurement fields. Recently, high-performance plastic scintillators or flexible film-type scintillators were developed by utilizing graphene and perovskite or 2D materials, as opposed to the Cd-series. Solution-processable halide perovskites have shown significant advantages for X-ray and γ-ray detection. As scintillators, halide perovskites showed high photo-luminescence quantum yields (PLQYs), controllable bandgaps, and short decay times, leading to excellent scintillation properties, including light yields and controllable luminescence wavelengths. To date, halide perovskites with high radio luminescence covering all visible regions have been demonstrated [
108]. In addition, research is being carried out that involves the doping of nanomaterials onto the surface by a method other than the previously widely used thermal polymerization method. Generally, base materials such as styrene, epoxy, and MPA are used when making plastic scintillators with added nanomaterials, and PPO and POPOP are frequently used as wavelength-shifting materials. Moreover, adding nanomaterials leads to different emission wavelengths depending on the size of the material, and with a large size of the nanomaterial, higher energy light is emitted. In QD-based scintillator research, when CdZnS/ZnS (CZS) nanomaterials were added to existing plastic materials, the resolution was twice as high as that of the NaI (Tl) scintillator [
77]. In addition, the analysis of photometric sensor development cases involving the use of nanomaterials as done here shows that many studies are being carried out in this area, including those focusing on the development of light sensors that are thin and flexible, not only on the development of light sensors that are not constrained by the measurement location, but also on the development of synthesis technology of inorganic perovskite nanomaterials with strong light-emitting properties and excellent photoluminescence characteristics. However, one of the problems with the addition of nanomaterials is that they are not uniformly polymerized or doped. It also takes a considerable amount of nanomaterials to show noticeable performance. Although nanomaterials are being studied globally, one problem is that nanomaterials are still expensive and lack mass-production technologies. Because production is limited to conventional synthesis methods, it is difficult to manufacture nanomaterials with certain properties. Cadmium-based nanomaterials have toxicity and environmental problems, as well as mass production problems, and while 2D materials are currently successful in large-size production, their quality levels remain low.
Nanomaterials are widely used in commercialized markets, and it is believed that it will be possible to realize the industrialization of nanomaterials if securing high-quality nanomaterial mass production technologies. In addition, nanomaterials can be used not only in semiconductor and display fields but also in radiation detection and measurement areas, meaning research on the production of large and high-performance scintillators and the application of nanomaterials at the science and technology stages is needed. In this contribution, we address the working mechanism of scintillators based on nanomaterials as well as the present advances toward nanomaterial-based scintillators in a wide range of applications. Challenges and prospects in future promising research directions for nanomaterial-based scintillators exist with a better understanding of a variety of methods for synthesis engineering, including the use of doping, and these are of great concerns to improve nanomaterials at high quality levels. There will also be the issues of the toxicity of nanomaterials and their stability in practical applications to be solved in the future for their adaptation in actual environments. We expect that this review will help communities and readers who study in these areas to understand the status and future challenges related to scintillation applications.