In Situ Catalytic Upgrading of Heavy Crude with CAPRI: Influence of Hydrogen on Catalyst Pore Plugging and Deactivation due to Coke
Abstract
:1. Introduction
2. Materials and Method
3. Results and Discussion
3.1. Upgraded Oil API Gravity and Viscosity
3.2. Effect of Hydrogen Pressure on Upgraded Oil API Gravity and Viscosity
3.3. Upgraded Oils True Boiling Point (TBP) Distribution
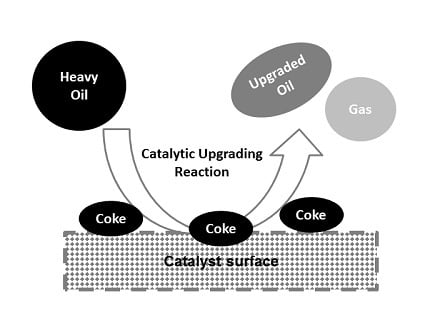
3.4. Upgraded Oil Asphaltene and Spent Catalyst Coke Contents
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
Data Access
Nomenclature
| THAI | Toe-to-Heel Air Injection |
| CAPRI | CAtalytc upgrading PRocess In situ |
| HDS | Hydrodesulphurisation |
| HDN | Hydrodenitrogenation |
| HDM | Hydrodemetallisation |
| TBP | True Boiling Point |
| IBP | Initial Boiling Point |
| TGA | Thermogravimetric Analysis |
| DTG | Differential Thermogravimetric |
| DVR | Degree of Viscosity Reduction |
| LHSV | Liquid Hourly Space Velocity |
| GHSV | Gas Hourly Space Velocity |
| WHSV | Weight Hourly Space Velocity |
| API | American Petroleum Institute |
| SEM | Scanning Electron Microscope |
References
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, K.S. Anaerobibiorefinery: Current status, challenges and opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Zitha, P.; Felder, R.; Zornes, D.; Brown, K.; Mohanty, K. Increasing Hydrocarbon Recovery Factors. 2011. Available online: http://www.spe.org/industry/increasing-hydrocarbon-recovery-factors.php (accessed on 13 November 2017).
- Hein, J.F. Geology of bitumen and heavy oil: An overview. J. Pet. Sci. Eng. 2017, 154, 551–563. [Google Scholar] [CrossRef]
- Upreti, S.R.; Lohi, A.; Kapadia, R.A.; El-Ha, R. Vapor extraction of heavy oil and bitumen: A review. Energy Fuels 2007, 21, 1562–1574. [Google Scholar] [CrossRef]
- Xia, T.M.; Greaves, M.; Werfilli, W.S.; Rathbone, R.R. Downhole conversion of Lloydminster heavy oil using THAI-CAPRI process. In Proceedings of the SPE/PS-CIM/CHOA International Thermal Operations and Heavy Oil Symposium and International Horizontal Well Technology Conference, Calgary, AB, Canada, 4–7 November 2002. [Google Scholar]
- Guo, K.; Zhang, Y.; Shi, Q.; Yu, Z. The effect of carbon-supported nickel nanoparticles in the reduction of carboxylic acids for in situ upgrading of heavy crude. Energy Fuels 2017. [Google Scholar] [CrossRef]
- Xia, T.X.; Greaves, M. 3-D physical model studies of downhole catalytic upgrading of Wolf Lake heavy oil using THAI. In Proceedings of the Petroleum Society’s Canadian International Petroleum Conference 2001, Calgary, AB, Canada, 12–14 June 2001. Paper 2001-17. [Google Scholar]
- Ayasse, C.; Greaves, M.; Turta, A. Oilfield In Situ Hydrocarbon Upgrading Process. U.S. Patent 6,412,557 B1, 2 July 2002. [Google Scholar]
- Hassanzadeh, H.; Abedi, J. Modelling and parameter estimation of ultra-dispersed in situ catalytic upgrading experiments in a batch reactor. Fuel 2010, 89, 2822–2828. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.P.; Leeke, G.A.; Wood, J.; Rigby, S.P.; Greaves, M. Experimental optimisation of catalytic process in situ for heavy-oil and bitumen upgrading. J. Can. Pet. Technol. 2011, 50, 33–47. [Google Scholar] [CrossRef]
- Galarraga, E.C.; Scott, C.; Loria, H.; Pereira-Almao, P. Kinetic models for upgrading Athabasca bitumen using unsupported NiWMo catalyst at low severity conditions. Ind. Eng. Chem. Res. 2012, 51, 140–146. [Google Scholar] [CrossRef]
- Hart, A.; Shah, A.; Leeke, G.; Greaves, M.; Wood, J. Optimization of the CAPRI process for heavy oil upgrading: Effect of hydrogen and guard bed. Ind. Eng. Chem. Res. 2013, 52, 15394–15406. [Google Scholar] [CrossRef]
- Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Downhole heavy crude oil upgrading using CAPRI: Effect of hydrogen and methane gases upon upgrading and coke formation. Fuel 2014, 119, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.; Wood, J.; Greaves, M. In Situ Catalytic Upgrading of Heavy Oil Using a Pelletized Ni-Mo/Al2O3 Catalyst in the THAI Process. J. Pet. Sci. Eng. 2017, 156, 958–965. [Google Scholar] [CrossRef]
- Hart, A.; Wood, J.; Greaves, M. Laboratory Investigation of CAPRI Catalytic THAI-add-on Process for Heavy Oil Production and In Situ Upgrading. J. Anal. Appl. Pyrolysis 2017. [Google Scholar] [CrossRef]
- Al-Marshed, A.; Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Effectiveness of Different Transition Metal Dispersed Catalysts for In Situ Heavy Oil Upgrading. Ind. Eng. Chem. Res. 2015, 54, 10645–10655. [Google Scholar] [CrossRef]
- Hart, A. Advanced Studies of Catalytic Upgrading of Heavy Oils. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Hart, A.; Leeke, G.; Greaves, M.; Wood, J. Downhole heavy crude oil upgrading using CAPRI: Effect of steam upon upgrading and coke formation. Energy Fuels 2014, 8, 1811–1819. [Google Scholar] [CrossRef]
- Hart, A.; Greaves, M.; Wood, J. A comparative study of fixed-bed and dispersed catalytic upgrading of heavy crude oil using-CAPRI. Chem. Eng. J. 2015, 282, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.; Lewis, C.; White, T.; Greaves, M.; Wood, J. Effect of cyclohexane as hydrogen-donor in ultradispersed catalytic upgrading of heavy oil. Fuel Process. Technol. 2015, 138, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Annual Report of the Petrobank Energy and Resources Ltd. 2007. Available online: https://www.knotia.ca/kstore/productinfo/fric08/PDFs/Petrobank%20Energy%20and%20Resources%20Ltd.%20AR_2007.pdf (accessed on 28 February 2018).
- Xia, T.X.; Greaves, M. Upgrading Athabasca Tar Sand Using Toe-to-Heel Air Injection. In Proceedings of the 2000 SPE/Petroleum Society of CIM International Conference on Horizontal Well Technology, Calgary, AB, Canada, 6–8 November 2000. [Google Scholar]
- Maity, S.K.; Ancheyta, J.; Marroquın, G. Catalytic Aquathermolysis Used for Viscosity Reduction of Heavy Crude Oils: A Review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Dobrynkin, N.M.; Batygina, M.V.; Noskov, A.S. Studies of Catalytic Properties of Inorganic Rock Matrices in Redox Reactions. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 5, 408–416. [Google Scholar] [CrossRef]
- Kim, J.-W.; Longstaff, C.D.; Hanson, V.F. Upgrading of bitumen-derived heavy oils over a commercial HDN catalyst. Fuel 1997, 76, 1143–1150. [Google Scholar] [CrossRef]
- Rankel, A.L. Hydroprocessing of Heavy Oil over CoMo/Carbon Supported Catalysts. Energy Fuels 1993, 7, 937–942. [Google Scholar] [CrossRef]
- Leyva, C.; Rana, S.M.; Trejo, F.; Ancheyta, J. On the Use of Acid-Base-Supported Catalysts for Hydroprocessing of Heavy Petroleum. Ind. Eng. Chem. Res. 2007, 46, 7448–7466. [Google Scholar] [CrossRef]
- Hsu, C.S.; Robinson, P.R. Practical Advances in Petroleum Processing; Springer: Berlin, Germany, 2006; Volume 1, pp. 23–34. [Google Scholar]
- Petrobank Encouraged by Early Combustion Operations at WHITESANDS THAITM Project. 2006. Available online: http://www.marketwired.com/press-release/petrobank-encouraged-by-early-combustion-operations-at-whitesands-thai-tm-project-tsx-pbg-611527.htm (accessed on 28 February 2018).
- Whitesands Project Performance Review. 2009. Available online: https://www.aer.ca/documents/oilsands/insitu presentations/2009AthabascaPetrobankWhitesandsTHAI9770.pdf (accessed on 28 February 2018).
- Ancheyta, J.; Betancourt, G.; Centeno, G.; Marroquın, G. Catalyst Deactivation during Hydroprocessing of Maya Heavy Crude Oil. (II) Effect of Temperature during Time-on-Stream. Energy Fuels 2003, 17, 462–467. [Google Scholar] [CrossRef]
- Brown, A.R.; Hart, A.; Coker, V.S.; Lloyd, J.R.; Wood, J. Upgrading of heavy oil by dispersed biogenic magnetite catalysts. Fuel 2016, 185, 442–448. [Google Scholar] [CrossRef]
- Wang, Y.; Yanling, C.; Jing, H.; Pei, L.; Chao, Y. Mechanism of catalytic aquathermolysis: Influences on heavy oil by two types of efficient catalytic Ions: Fe3+ and Mo6+. Energy Fuels 2010, 24, 1502–1510. [Google Scholar] [CrossRef]
- Sambi, I.S.; Khulbe, K.C.; Mann, R.S. Catalytic hydrotreatment of heavy gas oil. Ind. Eng. Chem. Prod. Dev. 1982, 21, 575–580. [Google Scholar] [CrossRef]
- Elizalde, I.; Rodriguez, A.M.; Ancheyta, J. Modeling the effect of pressure and temperature on the hydrocracking of heavy crude oil by the continuous kinetic lumping approach. Appl. Catal. A Gen. 2010, 382, 205–212. [Google Scholar] [CrossRef]
- Mapiour, M.; Sundaramurthy, V.; Dalai, A.K.; Adjaye, J. Effect of hydrogen partial pressure on hydrotreating of heavy gas oil derived from oil-sands bitumen: Experimental and kinetics. Energy Fuels 2010, 24, 772–784. [Google Scholar] [CrossRef]
- Cai, H.-Y.; Shaw, J.M.; Chung, K.H. Hydrogen solubility measurements in heavy oil and bitumen cuts. Fuel 2001, 80, 1055–1063. [Google Scholar] [CrossRef]
- Mapiour, M.; Sundaramurthy, V.; Dalai, A.K.; Adjaye, J. Effect of hydrogen purity on hydroprocessing of heavy gas oil derived from oil-sands bitumen. Energy Fuels 2009, 23, 2129–2135. [Google Scholar] [CrossRef]
- Rezaei, H.; Liu, X.; Ardakani, J.S.; Smith, J.K.; Bricker, M. A study of Cold Lake vacuum residue hydroconversion in batch and semi-batch reactors using unsupported MoS2 catalysts. Catal. Today 2010, 150, 244–254. [Google Scholar] [CrossRef]
- Longstaff, D.C.; Deo, M.D.; Hanson, F.V. Hydrotreatment of bitumen from the Whiterocks oil sands deposit. Fuel 1994, 73, 1523–1530. [Google Scholar] [CrossRef]
- Jarullah, T.A.; Mujtaba, M.I.; Wood, S.A. Improvement of the middle distillate yields during crude oil hydrotreatment in a trickle-bed reactor. Energy Fuels 2011, 25, 773–781. [Google Scholar] [CrossRef]
- Fathi, M.M.; Pereira-Almao, P. Kinetic modelling of Arab light vacuum residue upgrading by aquaprocessing at high space velocities. Ind. Eng. Chem. Res. 2013, 52, 612–623. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; Fu, Y.; Zhang, Y.; Gao, P.; Dai, C.; Zheng, K. Investigation on asphaltene structures during Venezuela heavy oil hydrocracking under various hydrogen pressures. Energy Fuels 2013, 27, 3692–3698. [Google Scholar] [CrossRef]
- Hart, A.; Omajali, J.B.; Murray, A.J.; Macaskie, L.E.; Greaves, M.; Wood, J. Comparison of the effects of dispersed noble metal (Pd) biomass supported catalysts with typical hydrogenation (Pd/C, Pd/Al2O3) and hydrotreatment catalysts (CoMo/Al2O3) for in-situ heavy oil upgrading with Toe-to-Heel Air Injection (THAI). Fuel 2016, 180, 367–376. [Google Scholar] [CrossRef]
- Zhang, X.; Shaw, M.J. Impact of multiphase behaviour on coke deposition in heavy oils hydroprocessing catalysts. Energy Fuels 2006, 20, 473–480. [Google Scholar] [CrossRef]
- Matsumura, A.; Kondo, T.; Sato, S.; Saito, K.; de Souza, W.F. Hydrocracking Brazilian Marlin vacuum residue with natural limonite. Part I: Catalytic activity of natural limonite. Fuel 2005, 84, 411–416. [Google Scholar] [CrossRef]
- Al-Mutairi, A.; Marafi, A. Effect of the operating pressure on residual oil hydroprocessing. Energy Fuels 2012, 26, 7257–7262. [Google Scholar] [CrossRef]
- Millan, M.; Adell, C.; Hinojosa, C.; Herod, A.A.; Kandiyoti, R. Mechanisms of catalytic activity in heavily coated hydrocracking catalysts. Oil Gas Sci. Technol. 2008, 63, 69–78. [Google Scholar] [CrossRef]
- Ovalles, C.; Rogel, E.; Moir, E.M.; Brait, A. Hydroprcessing of vacuum residues: Asphaltene characterisation and solvent extraction of spent slurry catalysts and the relationships with catalyst deactivation. Appl. Catal. A Gen. 2017, 532, 57–64. [Google Scholar] [CrossRef]
- Fumoto, E.; Sato, S.; Takanohashi, T. Characterization of an iron-oxide-based catalyst used for catalytic cracking of heavy oil with steam. Energy Fuels 2018. [Google Scholar] [CrossRef]









| Parameter | Value |
|---|---|
| Temperature (°C) | 425 |
| Pressure (bar) | 20–40 |
| Feed oil flow rate (mL·min−1) | 1 |
| Gas-to-oil ratio (mL·mL−1) | 200 |
| Residence time (min) | 6 |
| WHSV (h−1) | 9.1 |
| GHSV (h−1) | 2353 |
| LHSV (h−1) | 11.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, A.; Wood, J. In Situ Catalytic Upgrading of Heavy Crude with CAPRI: Influence of Hydrogen on Catalyst Pore Plugging and Deactivation due to Coke. Energies 2018, 11, 636. https://doi.org/10.3390/en11030636
Hart A, Wood J. In Situ Catalytic Upgrading of Heavy Crude with CAPRI: Influence of Hydrogen on Catalyst Pore Plugging and Deactivation due to Coke. Energies. 2018; 11(3):636. https://doi.org/10.3390/en11030636
Chicago/Turabian StyleHart, Abarasi, and Joseph Wood. 2018. "In Situ Catalytic Upgrading of Heavy Crude with CAPRI: Influence of Hydrogen on Catalyst Pore Plugging and Deactivation due to Coke" Energies 11, no. 3: 636. https://doi.org/10.3390/en11030636