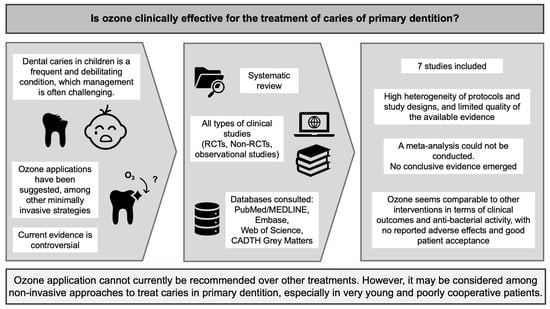
Ozone Treatment for the Management of Caries in Primary Dentition: A Systematic Review of Clinical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy and Study Selection
2.3. Data Extraction
2.4. Data Analysis
2.5. Risk of Bias Assessment
3. Results
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Risk of Bias Assessment
3.4. Synthesis of Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- Skeie, M.S.; Sen, A.; Dahllöf, G.; Fagerhaug, T.N.; Høvik, H.; Klock, K.S. Dental Caries at Enamel and Dentine Level among European Adolescents—A Systematic Review and Meta-Analysis. BMC Oral Health 2022, 22, 620. [Google Scholar] [CrossRef]
- Benelli, K.d.R.G.; Chaffee, B.W.; Kramer, P.F.; Knorst, J.K.; Ardenghi, T.M.; Feldens, C.A. Pattern of Caries Lesions and Oral Health-related Quality of Life throughout Early Childhood: A Birth Cohort Study. Eur. J. Oral Sci. 2022, 130, e12889. [Google Scholar] [CrossRef]
- Zaror, C.; Matamala-Santander, A.; Ferrer, M.; Rivera-Mendoza, F.; Espinoza-Espinoza, G.; Martínez-Zapata, M.J. Impact of Early Childhood Caries on Oral Health-related Quality of Life: A Systematic Review and Meta-analysis. Int. J. Dent. Hyg. 2022, 20, 120–135. [Google Scholar] [CrossRef]
- Duggal, M.; Gizani, S.; Albadri, S.; Krämer, N.; Stratigaki, E.; Tong, H.J.; Seremidi, K.; Kloukos, D.; BaniHani, A.; Santamaría, R.M.; et al. Best Clinical Practice Guidance for Treating Deep Carious Lesions in Primary Teeth: An EAPD Policy Document. Eur. Arch. Paediatr. Dent. 2022, 23, 659–666. [Google Scholar] [CrossRef]
- Leal, S.C. Minimal Intervention Dentistry in the Management of the Paediatric Patient. Br. Dent. J. 2014, 216, 623–627. [Google Scholar] [CrossRef]
- Sabbagh, H.J.; Albeladi, N.H.; Altabsh, N.Z.; Bamashmous, N.O. Risk Factors Associated with Children Receiving Treatment at Emergency Dental Clinics: A Case-Control Study. Int. J. Environ. Res. Public. Health 2023, 20, 1188. [Google Scholar] [CrossRef]
- Dahlander, A.; Soares, F.; Grindefjord, M.; Dahllöf, G. Factors Associated with Dental Fear and Anxiety in Children Aged 7 to 9 Years. Dent. J. 2019, 7, 68. [Google Scholar] [CrossRef]
- Desai, H.; Stewart, C.A.; Finer, Y. Minimally Invasive Therapies for the Management of Dental Caries—A Literature Review. Dent. J. 2021, 9, 147. [Google Scholar] [CrossRef]
- Innes, N.P.T.; Evans, D.J.P. Managing Caries in Primary Teeth. BDJ Team 2014, 1, 14118. [Google Scholar] [CrossRef]
- Santamaria, R.M.; Innes, N.P.T.; Machiulskiene, V.; Evans, D.J.P.; Splieth, C.H. Caries Management Strategies for Primary Molars: 1-Yr Randomized Control Trial Results. J. Dent. Res. 2014, 93, 1062–1069. [Google Scholar] [CrossRef]
- Conti, G.; Veneri, F.; Amadori, F.; Garzoni, A.; Majorana, A.; Bardellini, E. Evaluation of Antibacterial Activity of a Bioactive Restorative Material Versus a Glass-Ionomer Cement on Streptococcus Mutans: In-Vitro Study. Dent. J. 2023, 11, 149. [Google Scholar] [CrossRef]
- Veneri, F.; Bardellini, E.; Amadori, F.; Gobbi, E.; Belotti, R.; Majorana, A. Antibacterial Activity of New Hydrophilic Sealants: In Vitro Study. J. Indian. Soc. Pedod. Prev. Dent. 2020, 38, 387–392. [Google Scholar] [CrossRef]
- Gupta, N.; Chowdhary, N.; Reddy, V.R.; Nk, K.; Peddi, R.; Kumar, M. Evaluation of Caries Removal Efficacy Using BRIX 3000 and Atraumatic Restorative Treatment in Primary Molars: A Clinical Comparative Study. J. Contemp. Dent. Pract. 2022, 23, 419–424. [Google Scholar]
- Alkhouli, M.M.; Al Nesser, S.F.; Bshara, N.G.; AlMidani, A.N.; Comisi, J.C. Comparing the Efficacies of Two Chemo-Mechanical Caries Removal Agents (2.25% Sodium Hypochlorite Gel and Brix 3000), in Caries Removal and Patient Cooperation: A Randomized Controlled Clinical Trial. J. Dent. 2020, 93, 103280. [Google Scholar] [CrossRef]
- Contreras, V.; Toro, M.J.; Elías-Boneta, A.R.; Encarnación-Burgos, A. Effectiveness of Silver Diamine Fluoride in Caries Prevention and Arrest: A Systematic Literature Review. Gen. Dent. 2017, 65, 22–29. [Google Scholar]
- Splieth, C.H.; Banerjee, A.; Bottenberg, P.; Breschi, L.; Campus, G.; Ekstrand, K.R.; Giacaman, R.A.; Haak, R.; Hannig, M.; Hickel, R.; et al. How to Intervene in the Caries Process in Children: A Joint ORCA and EFCD Expert Delphi Consensus Statement. Caries Res. 2020, 54, 297–305. [Google Scholar] [CrossRef]
- Al-Yaseen, W.; Seifo, N.; Bhatia, S.; Innes, N. When Less Is More: Minimally Invasive, Evidence-Based Treatments for Dentine Caries in Primary Teeth—The Hall Technique and Silver Diamine Fluoride. Prim. Dent. J. 2021, 10, 33–42. [Google Scholar] [CrossRef]
- Santos, G.M.; Pacheco, R.L.; Bussadori, S.K.; Santos, E.M.; Riera, R.; De Oliveira Cruz Latorraca, C.; Mota, P.; Benavent Caldas Bellotto, E.F.; Martimbianco, A.L.C. Effectiveness and Safety of Ozone Therapy in Dental Caries Treatment: Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2020, 20, 101472. [Google Scholar] [CrossRef]
- Costa, T.; Linhares, D.; Ribeiro da Silva, M.; Neves, N. Ozone Therapy for Low Back Pain. A Systematic Review. Acta Reumatol. Port. 2018, 43, 172–181. [Google Scholar]
- Veneri, F.; Bardellini, E.; Amadori, F.; Conti, G.; Majorana, A. Efficacy of Ozonized Water for the Treatment of Erosive Oral Lichen Planus: A Randomized Controlled Study. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e675–e682. [Google Scholar] [CrossRef]
- Di Fede, O.; Del Gaizo, C.; Panzarella, V.; La Mantia, G.; Tozzo, P.; Di Grigoli, A.; Lo Casto, A.; Mauceri, R.; Campisi, G. Ozone Infiltration for Osteonecrosis of the Jaw Therapy: A Case Series. J. Clin. Med. 2022, 11, 5307. [Google Scholar] [CrossRef]
- Oliveira Modena, D.A.; de Castro Ferreira, R.; Froes, P.M.; Rocha, K.C. Ozone Therapy for Dermatological Conditions: A Systematic Review. J. Clin. Aesthet. Dermatol. 2022, 15, 65–73. [Google Scholar]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Y.; Wang, G.; Han, D. Benefits of Ozone on Mortality in Patients with COVID-19: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2023, 72, 102907. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Veneri, F.; Conti, G.; Majorana, A. Coronavirus Disease-2019 and Dental Practice: A Project on the Use of Ozonized Water in the Water Circuit of the Dental Armchair. Stomatologija 2020, 22, 35–38. [Google Scholar]
- Beretta, M.; Federici Canova, F. A New Method for Deep Caries Treatment in Primary Teeth Using Ozone: A Retrospective Study. Eur. J. Paediatr. Dent. 2017, 18, 111–115. [Google Scholar] [CrossRef]
- Celiberti, P.; Pazera, P.; Lussi, A. The Impact of Ozone Treatment on Enamel Physical Properties. Am. J. Dent. 2006, 19, 67–72. [Google Scholar]
- Dähnhardt, J.E.; Jaeggi, T.; Lussi, A. Treating Open Carious Lesions in Anxious Children with Ozone. A Prospective Controlled Clinical Study. Am. J. Dent. 2006, 19, 267–270. [Google Scholar]
- Crystal, Y.O.; Marghalani, A.A.; Ureles, S.D.; Wright, J.T.; Sulyanto, R.; Divaris, K.; Fontana, M.; Graham, L. Use of Silver Diamine Fluoride for Dental Caries Management in Children and Adolescents, Including Those with Special Health Care Needs. Pediatr. Dent. 2017, 39, 135–145. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Tong, A.; Flemming, K.; McInnes, E.; Oliver, S.; Craig, J. Enhancing Transparency in Reporting the Synthesis of Qualitative Research: ENTREQ. BMC Med. Res. Methodol. 2012, 12, 181. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Johansson, E.; Van Dijken, J.W.V.; Karlsson, L.; Andersson-Wenckert, I. Treatment Effect of Ozone and Fluoride Varnish Application on Occlusal Caries in Primary Molars: A 12-Month Study. Clin. Oral Investig. 2014, 18, 1785–1792. [Google Scholar] [CrossRef]
- Hauser-Gerspach, I.; Pfäffli-Savtchenko, V.; Dähnhardt, J.E.; Meyer, J.; Lussi, A. Comparison of the Immediate Effects of Gaseous Ozone and Chlorhexidine Gel on Bacteria in Cavitated Carious Lesions in Children In Vivo. Clin. Oral Investig. 2009, 13, 287–291. [Google Scholar] [CrossRef]
- Mese, M.; Tok, Y.T.; Kaya, S.; Akcay, M. Influence of Ozone Application in the Stepwise Excavation of Primary Molars: A Randomized Clinical Trial. Clin. Oral Investig. 2020, 24, 3529–3538. [Google Scholar] [CrossRef]
- Alhashmi, A.A.; Mohammed, R.A.K.; Hasan, M.S. An Innovative Technique for Treating Severe Caries in Primary Teeth with Ozone: A Retrospective Investigation. J. Pharm. Negat. Results 2023, 14, 1565–1568. [Google Scholar]
- Luppieri, V.; Manfra, A.; Ronfani, L.; Chermetz, M.; Cadenaro, M. Ozone Therapy for Early Childhood Caries (ECC) Treatment: An In Vivo Prospective Study. Appl. Sci. 2022, 12, 1964. [Google Scholar] [CrossRef]
- Ekstrand, K.R.; Ricketts, D.N.J.; Kidd, E.A.M.; Qvist, V.; Schou, S. Detection, Diagnosing, Monitoring and Logical Treatment of Occlusal Caries in Relation to Lesion Activity and Severity: An in Vivo Examination with Histological Validation. Caries Res. 1998, 32, 247–254. [Google Scholar] [CrossRef]
- Raafat Abdelaziz, R.; Mosallam, R.S.; Yousry, M.M. Tubular Occlusion of Simulated Hypersensitive Dentin by the Combined Use of Ozone and Desensitizing Agents. Acta Odontol. Scand. 2011, 69, 395–400. [Google Scholar] [CrossRef]
- Karlsson, L.; Kjaeldgaard, M. Ozone Treatment on Dentin Hypersensitivity Surfaces—A Pilot Study. Open Dent. J. 2017, 11, 65–70. [Google Scholar] [CrossRef]
- Rupel, K.; Ottaviani, G.; Bogdan Preda, M.T.; Poropat, A.; Gobbo, M.; DI Lenarda, R.; Biasotto, M. Ozone Treatment Combined with Sodium Fluoride for the Treatment of Dentin Hypersensitivity: An Exploratory Study. Minerva Dent. Oral Sci. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Meyfarth, S.; Cassano, K.; Warol, F.; Santos, M.D.D.; Scarparo, A. A New Efficient Agent to Chemo-Mechanical Caries Removal. Rev. Bras. Odontol. 2020, 77, 1. [Google Scholar] [CrossRef]
- Cardoso, M.; Coelho, A.; Lima, R.; Amaro, I.; Paula, A.; Marto, C.M.; Sousa, J.; Spagnuolo, G.; Marques Ferreira, M.; Carrilho, E. Efficacy and Patient’s Acceptance of Alternative Methods for Caries Removal—A Systematic Review. JCM 2020, 9, 3407. [Google Scholar] [CrossRef]
- Sajadi, F.S.; Rostamizadeh, M.; Hasheminejad, J.; Hasheminejad, N.; Borna, R.; Bazrafshani, M. Effect of Chlorhexidine, Fluoride and Green Tea Oral Gel on Pediatric Salivary Cariogenic Bacteria: A Clinical Trial Study. Int. J. Pediatr. 2021, 9, 13947–13956. [Google Scholar] [CrossRef]
- D’Amario, M.; Di Carlo, M.; Natale, S.M.; Memè, L.; Marzo, G.; Matarazzo, G.; Capogreco, M. Application of Ozone Therapy in Paediatric Dentistry. Appl. Sci. 2022, 12, 11100. [Google Scholar] [CrossRef]
- Veneri, F.; Vinceti, S.; Filippini, T. Fluoride and Caries Prevention: A Scoping Review of Public Health Policies. Ann. Ig. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Vinceti, S.R.; Veneri, F.; Filippini, T. Water Fluoridation between Public Health and Public Law: An Assessment of Regulations across Countries and Their Preventive Medicine Implications. Ann. Ig. 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- Lesaffre, E.; Philstrom, B.; Needleman, I.; Worthington, H. The Design and Analysis of Split-Mouth Studies: What Statisticians and Clinicians Should Know: THE SPLIT-MOUTH DESIGN. Statist. Med. 2009, 28, 3470–3482. [Google Scholar] [CrossRef]
- American Academy of Pediatric Dentistry Fluoride Therapy. In The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2023; pp. 352–358.
- Veneri, F.; Vinceti, M.; Generali, L.; Giannone, M.E.; Mazzoleni, E.; Birnbaum, L.S.; Consolo, U.; Filippini, T. Fluoride Exposure and Cognitive Neurodevelopment: Systematic Review and Dose-Response Meta-Analysis. Environ. Res. 2023, 221, 115239. [Google Scholar] [CrossRef]
- Iamandii, I.; De Pasquale, L.; Giannone, M.E.; Veneri, F.; Generali, L.; Consolo, U.; Birnbaum, L.S.; Castenmiller, J.; Halldorsson, T.I.; Filippini, T.; et al. Does Fluoride Exposure Affect Thyroid Function? A Systematic Review and Dose-Response Meta-Analysis. Environ. Res. 2024, 242, 117759. [Google Scholar] [CrossRef] [PubMed]
- Veneri, F.; Iamandii, I.; Vinceti, M.; Birnbaum, L.S.; Generali, L.; Consolo, U.; Filippini, T. Fluoride Exposure and Skeletal Fluorosis: A Systematic Review and Dose-Response Meta-Analysis. Curr. Envir Health Rep. 2023, 10, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.; Veneri, F.; Di Lorenzo, R.D.; Generali, L.; Vinceti, M.; Filippini, T. Fluoride Exposure and ADHD: A Systematic Review of Epidemiological Studies. Medicina 2023, 59, 797. [Google Scholar] [CrossRef]
- Fernandes, I.C.; Forte, F.D.S.; Sampaio, F.C. Molar-Incisor Hypomineralization (MIH), Dental Fluorosis, and Caries in Rural Areas with Different Fluoride Levels in the Drinking Water. Int. J. Paediatr. Dent. 2021, 31, 475–482. [Google Scholar] [CrossRef]
- Ismail, A.I.; Sohn, W. A Systematic Review of Clinical Diagnostic Criteria of Early Childhood Caries. J. Public Health Dent. 1999, 59, 171–191. [Google Scholar] [CrossRef]
- Ismail, A.I.; Pitts, N.B.; Tellez, M. Authors of the International Caries Classification and Management System (ICCMS) The International Caries Classification and Management System (ICCMSTM) An Example of a Caries Management Pathway. BMC Oral Health 2015, 15, S9. [Google Scholar] [CrossRef]
- Karlsson, L.; Johansson, E.; Tranæus, S. Validity and Reliability of Laser-Induced Fluorescence Measurements on Carious Root Surfaces in Vitro. Caries Res. 2009, 43, 397–404. [Google Scholar] [CrossRef]
- Kapor, S.; Rankovic, M.J.; Khazaei, Y.; Crispin, A.; Schüler, I.; Krause, F.; Lussi, A.; Neuhaus, K.; Eggmann, F.; Michou, S.; et al. Systematic Review and Meta-Analysis of Diagnostic Methods for Occlusal Surface Caries. Clin. Oral Investig. 2021, 25, 4801–4815. [Google Scholar] [CrossRef]
- Thanh, M.T.G.; Van Toan, N.; Toan, D.T.T.; Thang, N.P.; Dong, N.Q.; Dung, N.T.; Hang, P.T.T.; Anh, L.Q.; Tra, N.T.; Ngoc, V.T.N. Diagnostic Value of Fluorescence Methods, Visual Inspection and Photographic Visual Examination in Initial Caries Lesion: A Systematic Review and Meta-Analysis. Dent. J. 2021, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Sadatullah, S.; Mohamed, N.H.; Razak, F.A. The Antimicrobial Effect of 0.1 Ppm Ozonated Water on 24-Hour Plaque Microorganisms in Situ. Braz. Oral Res. 2012, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Thissera, B.; Mackay, W.; Rateb, M.E.; Yaseen, M. Bacterial and Fungal Disinfection via Ozonation in Air. J. Microbiol. Methods 2022, 194, 106431. [Google Scholar] [CrossRef]
- Gupta, S.; Deepa, D. Applications of Ozone Therapy in Dentistry. J. Oral Res. Rev. 2016, 8, 86–91. [Google Scholar] [CrossRef]
- International Scientific Committee of Ozone Therapy—ISCO3 Learning Methodology Instructions and Perfection in Ozone Therapy for Medical Doctors 2015. Available online: https://isco3.org/wp-content/uploads/2015/09/ISCO3-HUM-00-01.pdf (accessed on 20 January 2024).



| Database | Search Strategy |
|---|---|
| PubMed | (ozone[MH] OR ozone[tiab]) AND (“Dental Caries”[Mesh] OR “Dental Caries”[tiab]) AND humans[MH] |
| Embase | (‘ozone’/exp OR ‘ozone’) AND ‘dental caries’/exp AND ‘human’/exp |
| Web of Science | TS = (ozone) AND (TS = dental caries OR TI = dental caries OR AB = dental caries) AND TS = (human) |
| Reference | Study Design | Country | Participants | Ozone Treatment | Comparisons | Outcomes of Interest | Follow-up | Main Results |
|---|---|---|---|---|---|---|---|---|
| Alhashmi et al., 2023 | Retrospective cohort study | Iraq | 35 children (M:F = 19:16) Age range: 3–6 years Unit of analysis: extensive cavitated carious lesions of primary molars (n = 52) | Gaseous ozone, applied with single-patient silicone cup; applied after partial removal of carious dentin using round carbide burs, followed by composite restoration with rubber dam isolation. 4.7 g/m3 †, under vacuum, 60 s HealOzone a | n/a | Clinical success (not defined) | 3, 6, 12 months | At 12 months, clinical success was observed in 48/52 treated teeth (92.3%) |
| Beretta and Federici Canova; 2017 | Retrospective cohort study | Italy | 50 children (M:F = 28:22) Mean age: 5.8 ± 1.7 years Unit of analysis: extensive cavitated carious lesions of primary molars (n = 94) | Gaseous ozone, applied with single-patient silicone cup; applied after partial removal of carious dentin using round carbide burs, followed by composite restoration with rubber dam isolation. 32 g/m3, under vacuum, 60 s HealOzone X4 a | n/a | Clinical success as defined by the presence of all the following items: Restoration still in place. Absence of marginal microleakage. Absence of the restoration fractures. Presence of an interproximal contact (for Class II cavities). Absence of discoloration. Absence of pain Absence of pus or fistulas. | 3, 6, 12 months | At 12 months, clinical success was observed in 88/94 treated teeth (93.62%) |
| Dähnhardt et al., 2006 | Non-randomized controlled clinical trial (split-mouth) | Switzerland | 28 children (M:F = 9:23) Age range: 3–11 years Mean age 5.96 ± 2.36 years Unit of analysis: open single-surface carious lesion (n = 82) | Gaseous ozone applied after manual excavation to leathery consistency 4.7 g/m3, under vacuum, 20 s, a total of 5 times in 2-months intervals HealOzone a | Manual excavation to leathery consistency | Dentin hardness; Laser Fluorescence—LF (DIAGNOdent a); Parental and children attitude (questionnaire). | 0, 2, 4, 6, 8 months | Dentin hardness markedly increased in ozone group over time; no changes were observed in control group. LF values improved more in ozone group than in control group (non significant difference between groups). 93% of children reduced their dental anxiety following ozone treatment. No adverse effects were observed |
| Hauser-Gerspach et al., 2009 | Randomized controlled clinical trial | Switzerland | 40 children (M:F = 13:7) Age range: 2–8 years Mean age 5.1 years Unit of analysis: open single-surface carious lesion (n = 80) | Gaseous ozone applied on excavated/non excavated lesions 4.7 g/m3 (615 mL/min flow rate) under vacuum for 30 s HealOzone a | Chlorhexidine 1% gel applied on excavated/non excavated lesions for 30 s | Total bacterial count (CFU) | None (suitable restoration was placed after the experimental treatment) | Ozone application as well as 1% chlorhexidine gel application did not significantly reduce microorganisms count (CFU). The removal of decayed tissue showed no significant effect either. No adverse effects were observed |
| Johansson et al., 2014 | Randomized controlled clinical trial (split-mouth) 2 phases | Sweden | Total sample: 33 children; 50 pairs of carious lesions First phase: 11 children (M:F = 4:7) Mean age: 4.8 years Age range 3–6 years Unit of analysis: pairs of primary molars with cavitated caries lesions (visual inspection- VI score ≤ 3) (n = 15) Second phase: 22 children (M:F = 14:8) Mean age: 4.5 years Age range: 4–8 years Unit of analysis: pairs of primary molars with non-cavitated caries lesions (VI score ≤ 2a) (n = 35) | Gaseous ozone 4.7 g/m3 (615 mL/min flow rate), under vacuum, for 40 s at baseline, 3, 6, and 9 months or until failure (i.e., VI score 4) HealOzone a | Fluoride varnish 22,600 ppm NaF, applied in a thin layer with a micro-brush, at baseline, 3, 6, and 9 months or until failure (i.e., VI score 4) Duraphat® b | Visual Inspection (VI) according to Ekstrand score [44] Laser fluorescence (LF)- DIAGNOdent a | Baseline, 3, 6, 9, and 12 months or until failure (i.e., VI score 4) indicating necessity of an operative treatment. | Phase 1: In the 15 pairs VI ≤ 3 lesions, all of the lesions failed during the 12-month period (8 treated with ozone and 9 with fluoride). Median baseline LF values in the VI ≤ 3 group were 76 for ozone and 69 for fluoride group. At 12 months, they were 57 and 58, respectively. Phase 2: In the 35 pairs VI ≤ 2a lesions, one lesion failed (treated with ozone) after 12 months. Median baseline LF values were 21 and 19 for ozone and fluoride, respectively. At 12 months, median LF values were 15 for ozone group and 17.5 for fluoride–varnish group. No difference in LF values was observed over time within and between groups. In cavitated lesions, neither ozone nor fluoride varnish treatments stopped the progression of caries. Non-cavitated lesions showed slight or no progression following both treatments. No adverse effects were observed |
| Luppieri et al., 2022 | Non-controlled clinical trial | Italy | 20 children with ECC (M:F = 9:11) Age range: 3–5 years Mean age 4.5 Unit of analysis: decayed tooth (n = 20; 9 molars, 8 incisors, and 1 canine) | Gaseous ozone; application on cleaned tooth (rotating low-speed brush) using ozone intensity program n.6 (of 10), 0.4 g/m3 †, for 60 s, keeping the probe’s tip in continuous motion and perpendicular to the decayed surface at 1 mm from it; one application per week, for four consecutive weeks. OzoneDTA® c | n/a | Dentin compactness Dentin hypersensitivity to thermal stimuli (air syringe spray) according to the Wong–Baker Faces Pain Rating Scale (WBFPRS) Salivary Bacterial Count (Streptococcus mutans) at T0 and T1 Patients’ and families’ quality of life—Early Childhood Oral Health Impact Scale (ECOHIS) at T0 and T1 | T0 = baseline; T1 = after the 4-sessions ozone treatment cycle; T2 = 1 month from T1; T3 = 2 months from T1, T4 = 3 months from T1. | Affected dentin compactness significantly improved from T0 to T1. A mild improvement (not statistically significant) was detected at the other time points. 2 children reported hypersensitivity/pain at baseline, with a mean reported value of 3.5 according to the WBFPRS. At T1, none of the patients reported dentin hypersensitivity; no further changes were observed at later follow-ups. S. mutans count (n = 11) decreased significantly from T0 to T1. 8 of the 9 patients that were positive at T0 became negative at T1, while no changes were detected for the negative ones. The mean ECOHIS score was 9.4 at T0 and was 5.9 at T1 |
| Mese et al., 2020 | Randomized controlled clinical trial | Turkey | 105 children (M:F = 46:59) Age range: 6–10 Unit of analysis: deep caries lesion of lower primary molars (n = 105) | Gaseous ozone (n = 35), applied after partial removal of carious dentin (stepwise excavation), 4.7 g/m3, under vacuum, for 60 s HealOzone a | Partial removal of carious dentin (stepwise excavation) followed by: I.CHX 2% solution (n = 35) applied once for 60 s using a brush II. Control—no disinfectant treatment (n = 35) In all 3 groups, Ca(OH)2 liner was placed on the remaining carious dentin and the tooth was temporarily restored. | Clinical evaluation of dentin color, consistency, and humidity Total and specific bacterial counts (CFUs) for Streptococcus mutans and Lactobacillus spp., on dentine sample | Immediately after treatment and after 4 months (at replacement of temporary restoration) T0: immediately after partial removal of necrotic carious dentin T1: immediately after applying the disinfection procedure T2: 4-month follow-up (immediately after the removal of the temporary restoration) T3: At the second appointment, after complete removal of the remaining carious dentin. | Total incidence of pulp exposure was similar among groups (3.03% in the control group, 3.125% in the CHX group, and 2.85% in the ozone group). Dentin became gradually dryer and harder in all the groups, and there was no significant difference among the groups both between T0 and T2, and T0 and T3. Dentin color became darker at the second appointment in the CHX and ozone groups, with significant differences as compared to control group, but no significant difference among treatment groups. The reduction percentages of the total number of microorganism species were significantly higher in the CHX and ozone groups compared to the control group. CHX resulted in significantly higher reduction than ozone between T0-T1 and T0-T2. Total CFU showed no significant difference among groups between T0 and T3. With regards to S. mutans and Lactobacillus spp. CFUs, CHX was significantly more effective than ozone between T0–T1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veneri, F.; Filippini, T.; Consolo, U.; Vinceti, M.; Generali, L. Ozone Treatment for the Management of Caries in Primary Dentition: A Systematic Review of Clinical Studies. Dent. J. 2024, 12, 69. https://doi.org/10.3390/dj12030069
Veneri F, Filippini T, Consolo U, Vinceti M, Generali L. Ozone Treatment for the Management of Caries in Primary Dentition: A Systematic Review of Clinical Studies. Dentistry Journal. 2024; 12(3):69. https://doi.org/10.3390/dj12030069
Chicago/Turabian StyleVeneri, Federica, Tommaso Filippini, Ugo Consolo, Marco Vinceti, and Luigi Generali. 2024. "Ozone Treatment for the Management of Caries in Primary Dentition: A Systematic Review of Clinical Studies" Dentistry Journal 12, no. 3: 69. https://doi.org/10.3390/dj12030069