Coating Mechanism of AuNPs onto Sepiolite by Experimental Research and MD Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Studies
2.2. Zeta Potential Measurements
2.3. Characterization Analyses
2.4. Computational Details
3. Results and Discussion
3.1. Experimental Studies
3.2. Characterization Analyses
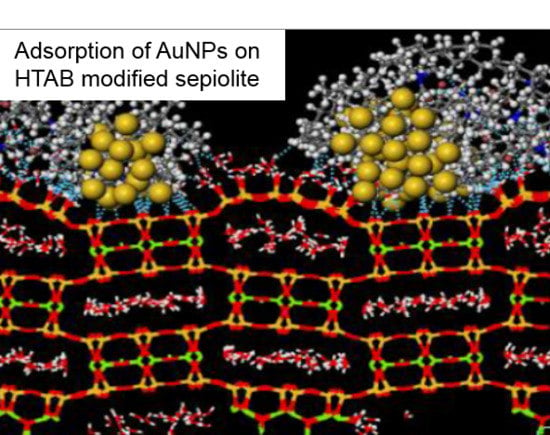
3.3. MD Simulations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, J.D.; Blanchard, G.J. Formation of gold nanoparticles using amine reducing agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Pecharromán, C.; Esteban-Cubillo, A.; Fernández, H.; Esteban-Tejeda, L.; Pina-Zapardiel, R.; Moya, J.S.; Solis, J.; Afonso, C.N. Synthesis, conforming, linear, and non-linear optical properties of gold nanoparticles-sepiolite compacts. Plasmonics 2009, 4, 261–266. [Google Scholar] [CrossRef]
- Tiwari, N.; Kalele, S.; Kulkarni, S.K. Modulation of optical properties of gold nanorods on addition of KOH. Plasmonics 2007, 2, 231–236. [Google Scholar] [CrossRef]
- Zhang, R.; Hummelgard, M.; Olin, H. Simple synthesis of clay-gold nanocomposites with tunable color. Langmuir 2010, 26, 5823–5828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Letaief, S.; Liu, Y.; Gervais, F.; Detellier, C. Clay mineral-supported gold nanoparticles. Appl. Clay Sci. 2009, 43, 439–446. [Google Scholar] [CrossRef]
- Ung, T.; Liz-Marzán, L.M.; Mulvaney, P. Optical properties of thin films of Au@SiO2 particles. J. Phys. Chem. B 2001, 105, 3441–3452. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Karataş, D.; Senol-Arslan, D.; Ozdemir, O. Experimental and atomistic modeling of adsorption of azo acid 57 on sepiolite. Clays Clay Miner. 2018. [Google Scholar] [CrossRef]
- Ozdemir, O.; Armagan, B.; Turan, M.; Çelik, M.S. Comparison of the adsorption characteristics of azo-reactive dyes on mezoporous minerals. Dye. Pigment. 2004, 62, 49–60. [Google Scholar] [CrossRef]
- Ozdemir, O.; Cinar, M.; Sabah, E.; Arslan, F.; Celik, M.S. Adsorption of anionic surfactants onto sepiolite. J. Hazard. Mater. 2007, 147, 625–632. [Google Scholar] [CrossRef]
- Sabah, E.; Çelik, M.S. Adsorption mechanism of quaternary amines by sepiolite. Sep. Sci. Technol. 2002, 37, 3081–3097. [Google Scholar] [CrossRef]
- Sabah, E.; Çinar, M.; Çelik, M.S. Decolorization of vegetable oils: Adsorption mechanism of β-carotene on acid-activated sepiolite. Food Chem. 2007, 100, 1661–1668. [Google Scholar] [CrossRef]
- Belova, V.; Andreeva, D.V.; Möhwald, H.; Shchukin, D.G. Ultrasonic intercalation of gold nanoparticles into clay matrix in the presence of surface-active materials. Part I: Neutral polyethylene glycol. J. Phys. Chem. C 2009, 113, 5381–5389. [Google Scholar] [CrossRef]
- Belova, V.; Möhwald, H.; Shchukin, D.G. Ultrasonic intercalation of gold nanoparticles into a clay matrix in the presence of surface-active materials. Part II: Negative sodium dodecylsulfate and positive cetyltrimethylammonium bromide. J. Phys. Chem. C 2009, 113, 6751–6760. [Google Scholar] [CrossRef]
- Chenouf, M.; Megías-Sayago, C.; Ammari, F.; Ivanova, S.; Centeno, M.; Odriozola, J. Immobilization of stabilized gold nanoparticles on various ceria-based oxides: Influence of the protecting agent on the glucose oxidation reaction. Catalysts 2019, 9, 125. [Google Scholar] [CrossRef]
- Yadav, D.K.; Gupta, R.; Ganesan, V.; Sonkar, P.K. Individual and simultaneous voltammetric determination of ascorbic acid, uric acid and folic acid by using a glassy carbon electrode modified with gold nanoparticles linked to bentonite via cysteine groups. Microchim. Acta 2017, 184, 1951–1957. [Google Scholar] [CrossRef]
- Letaief, S.; Pell, W.; Detellier, C. Deposition of gold nanoparticles on organo-kaolinite—Application in electrocatalysis for carbon monoxide oxidation. Can. J. Chem. 2011, 89, 845–853. [Google Scholar] [CrossRef]
- Philip, A.; Lihavainen, J.; Keinänen, M.; Pakkanen, T.T. Gold nanoparticle-decorated halloysite nanotubes —Selective catalysts for benzyl alcohol oxidation. Appl. Clay Sci. 2017, 143, 80–88. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Phadtare, S.; Vinod, V.P.; Kumar, A.; Rao, M.; Chaudhari, R.V.; Sastry, M. Gold nanoparticles assembled on amine-functionalized Na−Y zeolite: A biocompatible surface for enzyme immobilization. Langmuir 2003, 19, 3858–3863. [Google Scholar] [CrossRef]
- Letaief, S.; Grant, S.; Detellier, C. Phenol acetylation under mild conditions catalyzed by gold nanoparticles supported on functional pre-acidified sepiolite. Appl. Clay Sci. 2011, 53, 236–243. [Google Scholar] [CrossRef]
- Pecharromán, C.; Esteban-Cubillo, A.; Montero, I.; Moya, J.S.; Aguilar, E.; Santarén, J.; Alvarez, A. Monodisperse and corrosion-resistant metallic nanoparticles embedded into sepiolite particles for optical and magnetic applications. J. Am. Ceram. Soc. 2006, 89, 3043–3049. [Google Scholar] [CrossRef]
- Tiemblo, P.; Benito, E.; García, N.; Esteban-Cubillo, A.; Pina-Zapardiel, R.; Pecharromán, C. Multiscale gold and silver plasmonic plastics by melt compounding. RSC Adv. 2012, 2, 915–919. [Google Scholar] [CrossRef]
- Liu, M.; Pu, M.; Ma, H. Preparation, structure and thermal properties of polylactide/sepiolite nanocomposites with and without organic modifiers. Compos. Sci. Technol. 2012, 72, 1508–1514. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Zhang, G. Core-shell Ag@Pt nanoparticles supported on sepiolite nanofibers for the catalytic reduction of nitrophenols in water: Enhanced catalytic performance and DFT study. Appl. Catal. B: Environ. 2017, 205, 262–270. [Google Scholar] [CrossRef]
- Rebitski, E.P.; Alcântara, A.C.S.; Darder, M.; Cansian, R.L.; Gómez-Hortigüela, L.; Pergher, S.B.C. Functional carboxymethylcellulose/zein bionanocomposite films based on neomycin supported on sepiolite or montmorillonite clays. ACS Omega 2018, 3, 13538–13550. [Google Scholar] [CrossRef]
- Fitaroni, L.B.; Venâncio, T.; Tanaka, F.H.; Gimenez, J.C.F.; Costa, J.A.S.; Cruz, S.A. Organically modified sepiolite: Thermal treatment and chemical and morphological properties. Appl. Clay Sci. 2019, 179, 105149. [Google Scholar] [CrossRef]
- Sabah, E.; Turan, M.; Çelik, M.S. Adsorption mechanism of cationic surfactants onto acid- and heat-activated sepiolites. Water Res. 2002, 36, 3957–3964. [Google Scholar] [CrossRef]
- da Silva, J.; Dias, R.; da Hora, G.; Soares, T.; Meneghetti, M. Molecular dynamics simulations of cetyltrimethylammonium bromide (CTAB) micelles and their interactions with a gold surface in aqueous solution. J. Braz. Chem. Soc. 2017. [Google Scholar] [CrossRef]
- de Barros, H.R.; Piovan, L.; Sassaki, G.L.; de Araujo Sabry, D.; Mattoso, N.; Nunes, A.M.; Meneghetti, M.R.; Riegel-Vidotti, I.C. Surface interactions of gold nanorods and polysaccharides: From clusters to individual nanoparticles. Carbohydr. Polym. 2016, 152, 479–486. [Google Scholar] [CrossRef]
- Meena, S.K.; Celiksoy, S.; Schafer, P.; Henkel, A.; Sonnichsen, C.; Sulpizi, M. The role of halide ions in the anisotropic growth of gold nanoparticles: A microscopic, atomistic perspective. Phys. Chem. Chem. Phys. 2016, 18, 13246–13254. [Google Scholar] [CrossRef]
- Meena, S.K.; Sulpizi, M. Understanding the microscopic origin of gold nanoparticle anisotropic growth from molecular dynamics simulations. Langmuir 2013, 29, 14954–14961. [Google Scholar] [CrossRef] [PubMed]
- Sau, T.K.; Murphy, C.J. Self-assembly patterns formed upon solvent evaporation of aqueous cetyltrimethylammonium bromide-coated gold nanoparticles of various shapes. Langmuir 2005, 21, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Armagan, B.; Ozdemir, O.; Turan, M.; Celik, M.S. Adsorption of negatively charged azo dyes onto surfactant-modified sepiolite. J Environ. Eng. 2003, 129, 709–715. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Materials Studio. 16.1.0.21; Dassault Systèmes BIOVIA: San Diego, CA, USA, 2016. [Google Scholar]
- Post, J.E.; Bish, D.L.; Heaney, P.J. Synchrotron powder X-ray diffraction study of the structure and dehydration behavior of sepiolite. Am. Mineral. 2007, 92, 91–97. [Google Scholar] [CrossRef]
- Dauber-Osguthorpe, P.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins 1988, 4, 31–47. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Jaishankar, A.; Jusufi, A.; Vreeland, J.L.; Deighton, S.; Pellettiere, J.; Schilowitz, A.M. Adsorption of stearic acid at the iron oxide/oil interface: Theory, experiments, and modeling. Langmuir 2019, 35, 2033–2046. [Google Scholar] [CrossRef]
- Karataş, D.; Tekin, A.; Bahadori, F.; Çelik, M.S. Interaction of curcumin in a drug delivery system including a composite with poly(lactic-co-glycolic acid) and montmorillonite: A density functional theory and molecular dynamics study. J. Mater. Chem. B 2017, 5, 8070–8082. [Google Scholar] [CrossRef]
- Li, J.-W.; Zhang, S.-H.; Gou, R.-J.; Han, G.; Chen, M.-H. The effect of crystal-solvent interaction on crystal growth and morphology. J. Cryst. Growth 2019, 507, 260–269. [Google Scholar] [CrossRef]
- Li, W.; Pang, X.; Snape, C.; Zhang, B.; Zheng, D.; Zhang, X. Molecular simulation study on methane adsorption capacity and mechanism in clay minerals: Effect of clay type, pressure, and water saturation in shales. Energy Fuels 2019, 33, 765–778. [Google Scholar] [CrossRef]
- Rahmani, F.; Nouranian, S. Thermal analysis of montmorillonite/graphene double-layer coating as a potential lightning strike protective layer for cross-linked epoxy by molecular dynamics simulation. ACS Appl. Nano Mater. 2018, 1, 2521–2525. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kawaguchi, D.; Sasahara, K.; Inutsuka, M.; Yamamoto, S.; Uchida, K.; Mita, K.; Ogawa, H.; Takenaka, M.; Tanaka, K. Aggregation states of poly(4-methylpentene-1) at a solid interface. Polym. J. 2018, 51, 247–255. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Sun, D.; Li, Y.; Wu, T. Effective removal of emulsified oil from oily wastewater using surfactant-modified sepiolite. Appl. Clay Sci. 2018, 157, 227–236. [Google Scholar] [CrossRef]
- Karataş, D.; Tekin, A.; Çelik, M.S. Adsorption of quaternary amine surfactants and their penetration into the intracrystalline cavities of sepiolite. New J. Chem. 2013, 37, 3936. [Google Scholar] [CrossRef]
- Garcia-Romero, E.; Suarez, M. Sepiolite-palygorskite polysomatic series: Oriented aggregation as a crystal growth mechanism in natural environments. Am. Mineral. 2014, 99, 1653–1661. [Google Scholar] [CrossRef] [Green Version]
- Vivaldi, J.L.M.; Robertson, R. Palygorskite and sepiolite (the hormites). In The Electron-Optical Investigation of Clays; Gard, J.A., Ed.; The Mineralogical Society: London, UK, 1971; Volume 41, pp. 255–257. [Google Scholar]
- Suárez, M.; García-Romero, E. Variability of the surface properties of sepiolite. Appl. Clay Sci. 2012, 67–68, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Sastry, M.; Rao, M.; Ganesh, K.N. Electrostatic assembly of nanoparticles and biomacromolecules. Acc. Chem. Res. 2002, 35, 847–855. [Google Scholar] [CrossRef]












| Compound | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | TiO2 | P2O5 | MnO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| % | 44.42 | 2.36 | 0.95 | 19.87 | 2.00 | 0.33 | 0.13 | 0.01 | 0.01 | 29.76 |
| Models | Monolayer CTAB/SEP | Bilayer CTAB/SEP | AuNPs/SEP | Monolayer AuNPs/SEP | Bilayer AuNPs/SEP | |
|---|---|---|---|---|---|---|
| Pairs | ||||||
| AuNPs/SEP | N/A | −443.39 | −320.28 | −242.69 | ||
| −443.39 1 | −320.29 1 | −242.69 1 | ||||
| 0.00 2 | 0.00 2 | 0.00 2 | ||||
| AuNPs/CTAB | N/A | −484.26 | −526.18 | |||
| −484.26 1 | −526.19 1 | |||||
| 0.00 2 | 0.00 2 | |||||
| CTAB/SEP | −1373.63 | −1630.76 | N/A | −1011.11 | −1575.93 | |
| −99.88 1 | −126.37 1 | −83.30 1 | −125.95 1 | |||
| −1273.75 2 | −1504.40 2 | −927.81 2 | −1449.98 2 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karataş, D.; Senol Arslan, D.; Kursun Unver, I.; Ozdemir, O. Coating Mechanism of AuNPs onto Sepiolite by Experimental Research and MD Simulation. Coatings 2019, 9, 785. https://doi.org/10.3390/coatings9120785
Karataş D, Senol Arslan D, Kursun Unver I, Ozdemir O. Coating Mechanism of AuNPs onto Sepiolite by Experimental Research and MD Simulation. Coatings. 2019; 9(12):785. https://doi.org/10.3390/coatings9120785
Chicago/Turabian StyleKarataş, Deniz, Dilek Senol Arslan, Ilgin Kursun Unver, and Orhan Ozdemir. 2019. "Coating Mechanism of AuNPs onto Sepiolite by Experimental Research and MD Simulation" Coatings 9, no. 12: 785. https://doi.org/10.3390/coatings9120785