Kinetic Modeling of Grain Boundary Diffusion: Typical, Bi-Modal, and Semi-Lamellar Polycrystalline Coating Morphologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Numerical Model
- Fick’s laws of diffusion are valid both in grain and GB areas;
- Volumetric diffusion from surface to deeper layers of the coating;
- Diffusion from GB to grain, and vice versa;
- Diffusion along and across GB;
- Chemical homogeneity at the interfaces, with no element segregation and/or precipitation;
- The diffusivity in the grain boundary is much higher than the diffusivity in the bulk;
- Diffusion coefficients are independent of time but dependent on position;
- Initial zero concentration of diffusing atoms in the whole volume;
- The Dirichlet boundary condition for the first layer: ;
- The Neumann condition on all other sides: .
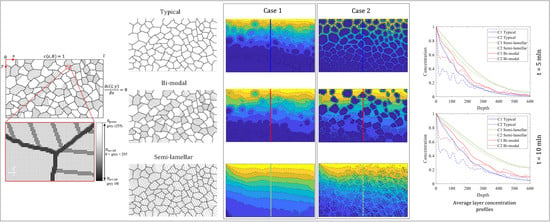
2.2. Polycrystalline Microstructures
3. Results
3.1. Morphology Influence on Diffusion Regimes
3.2. Influence of Polycrystal Morphology on the Overall Rate
4. Conclusions
- Different morphologies having the same values of diffusion coefficients Dg and Dgb can provide a variety of different diffusion regimes. The diffusion regime depends not only on the total diffusivity and ratio Dgb/Dg, but also on the morphological features of the coating:
- ○
- A dense network of primary and secondary GBs facilitates diffusion, and the Type-A regime is favored.
- ○
- Type-B regime in realistic morphologies was not observed individually in the simulated coatings. It was complemented by Type-A regime in the region by the surface, the depth of which increased with time. Additionally, if coating includes grains of significantly different average diffusivity (bi-modal in our case, containing grains both with and without secondary GBs), Type-A regime might replace Type-B in grains with higher diffusivity (presence of secondary GBs).
- ○
- Type-C regime could only be observed in coatings without secondary GBs in the simulated cases, showing that secondary GBs are effective diffusion and grain saturation pathways.
- ○
- In realistic complex morphology, all three regimes occur simultaneously in different places of the material depending on the local features of the microstructure.
- While the introduction of fast GBs accelerates diffusion, the level of acceleration depends on the morphological features, and ultimately, on the deposition method and conditions, since fast pathways and diffusion flux areas are limited to GBs, which reduces the effectiveness of total diffusivity compared to monocrystal diffusion. GB distribution and connections are important to the mass transfer process, as they accelerate diffusant transport locally.
- ○
- Speed-up of diffusion due to GBs does not straightforwardly depend on the total or GB diffusivities Dtotal and Dgb, but on the features of the coating morphology, which can provide more or less weight to the influence of one or another.
- ○
- Even in polycrystal coatings, the overall process is dominated by grain diffusion for most of the transient. Only initially is GB diffusion responsible for the larger part of the overall uptake of the diffusant. However, the denser network of GBs is present, the longer GB diffusion can dominate, and the larger fraction of the overall rate it takes than grain diffusion is dominating.
- ○
- The ratio of average grain and GB diffusion rates in the coating is constant for most of the transient and is dependent on the morphology. However, it corresponds directly neither to the ratio of grain and GB volumes, nor to the average GB-like diffusivity, expressed through the coefficient agb.
- ○
- If the coatings differ only by the presence of secondary GBs, developed diffusion in them maintains the same ratio of average rates at the same average concentrations, regardless of the irregular nature of the microstructures.
- ○
- The overall diffusion rate is generally dependent on the Dgb/Dg ratio (constant Dgb). However, the level of this dependence depends on the morphology. In the coatings with a high number of GBs, e.g., semi-lamellar, the relative influence of Dg is marginal.
- ○
- More densely located secondary grain boundaries create areas of faster diffusion (or accumulation areas), and a lower Dgb/Dg ratio leads to greater diffusant leakage from GBs to grains and a more uniform concentration distribution in the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.; Park, S.J. Deformation of single crystals, polycrystalline materials, and thin films: A Review. Materials 2003, 12, 2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.K.; On, N.; Choi, C.H.; Kim, M.J.; Kang, S.; Lim, J.H.; Jeong, K.J. Polycrystalline indium gallium tin oxide thin-film transistors with high mobility exceeding 100 cm 2/Vs. IEEE Electron Device Lett. 2021, 42, 347–350. [Google Scholar] [CrossRef]
- Li, G.; Rahim, M.Z.; Pan, W.; Wen, C.; Ding, S. The manufacturing and the application of polycrystalline diamond tools–A comprehensive review. J. Manuf. Process. 2020, 56, 400–416. [Google Scholar] [CrossRef]
- Angadi, R.V.; Revanasiddesh, B.; Vineet Kumar, P.K. A review on different types of materials employed in solar photovoltaic panel. Int. J. Eng. Res. Technol. 2019, 7. [Google Scholar]
- Ryu, S.; Yu, W.; Chang, I.; Park, T.; Cho, G.Y.; Cha, S.W. Three dimensional YSZ interface engineering layer for enhancement of oxygen reduction reactions of low temperature solid oxide fuel cells. Ceram. Int. 2020, 46, 12648–12655. [Google Scholar] [CrossRef]
- Pan, H.; Yin, X.; Xue, J.; Cheng, L.; Zhang, L. In-situ synthesis of hierarchically porous and polycrystalline carbon nanowires with excellent microwave absorption performance. Carbon 2016, 107, 36–45. [Google Scholar] [CrossRef]
- Magari, Y.; Kataoka, T.; Yeh, W.; Furuta, M. High-mobility hydrogenated polycrystalline In2O3 (In2O3: H) thin-film transistors. Nat. Commun. 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Conte, M.; Aktaa, J. Manufacturing influences on microstructure and fracture mechanical properties of polycrystalline tungsten. Nucl. Mater. Energy 2019, 21, 100591. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.H.; Lee, S.Y.; Lee, J.; Cho, S.O. A Transparent Nano-Polycrystalline ZnWO4 Thin-Film Scintillator for High-Resolution X-ray Imaging. ACS Omega 2021, 6, 33224–33230. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L. Origin and future of polycrystalline ceramic lasers. IEEE J. Sel. Top. Quantum Electron. 2018, 24, 1–7. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Hopper, T.R.; Bakulin, A.A.; Yip, H.L. Materials, photophysics and device engineering of perovskite light-emitting diodes. Rep. Prog. Phys. 2021, 84, 046401. [Google Scholar] [CrossRef] [PubMed]
- Wieghold, S.; Correa-Baena, J.P.; Nienhaus, L.; Sun, S.; Shulenberger, K.E.; Liu, Z.; Tresback, J.S.; Shin, S.S.; Bawendi, M.G.; Buonassisi, T. Precursor concentration affects grain size, crystal orientation, and local performance in mixed-ion lead perovskite solar cells. ACS Appl. Energy Mater. 2018, 1, 6801–6808. [Google Scholar] [CrossRef]
- Choi, H.; Choi, K.; Choi, Y.; Kim, T.; Lim, S.; Park, T. A Review on Reducing Grain Boundaries and Morphological Improvement of Perovskite Solar Cells from Methodology and Material-Based Perspectives. Small Methods 2020, 4, 1900569. [Google Scholar] [CrossRef]
- Zheng, R.; Du, J.P.; Gao, S.; Somekava, H.; Ogata, S.; Tsuji, N. Transition of dominant deformation mode in bulk polycrystalline pure Mg by ultra-grain refinement down to sub-micrometer. Acta Mater. 2020, 198, 35–46. [Google Scholar] [CrossRef]
- Jia, H.; Liu, X.; Li, Z.; Sun, S.; Li, M. The effect of grain size on the deformation mechanisms and mechanical properties of polycrystalline TiN: A molecular dynamics study. Comput. Mater. Sci. 2018, 143, 189–194. [Google Scholar] [CrossRef]
- Šalkus, T.; Kazakevičius, E.; Banys, J.; Kranjčec, M.; Chomolyak, A.A.; Neimet, Y.Y.; Studenyak, I.P. Influence of grain size effect on electrical properties of Cu6PS5I superionic ceramics. Solid State Ion. 2014, 262, 597–600. [Google Scholar] [CrossRef]
- Xue, Q.; Huang, Z.; Zhang, J.; Zhang, H.; Feng, Z. Grain boundary segregation and its influences on ionic conduction properties of scandia doped zirconia electrolytes. J. Rare Earths 2019, 37, 645–651. [Google Scholar] [CrossRef]
- Huo, D.; Baldinozzi, G.; Simeone, D.; Khodja, H.; Surble, S. Grain size-dependent electrical properties of La1. 95Sr0. 05Zr2O7-δ as potential Proton Ceramic Fuel Cell electrolyte. Solid State Ion. 2016, 298, 35–43. [Google Scholar] [CrossRef]
- Lee, W.; Kihm, K.D.; Kim, H.G.; Shim, S.; Lee, C.; Park, J.S.; Cheon, S.; Kwon, O.M.; Lim, G.; Lee, W. In-plane thermal conductivity of polycrystalline chemical vapor deposition graphene with controlled grain sizes. Nano lett. 2017, 17, 2361–2366. [Google Scholar] [CrossRef]
- Gur, S.; Sadat, M.R.; Frantziskonis, G.N.; Bringuier, S.; Zhang, L.; Muralidharan, K. The effect of grain-size on fracture of polycrystalline silicon carbide: A multiscale analysis using a molecular dynamics-peridynamics framework. Comput. Mater. Sci. 2019, 159, 341–348. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Han, Y.; Zhao, L.; Xiao, B. New perspectives on the grain boundary misorientation angle dependent intergranular corrosion of polycrystalline nickel-based 625 alloy. Corros. Sci. 2020, 172, 108718. [Google Scholar] [CrossRef]
- Li, Z.-P.; Mori, T.; Auchterlonie, G.J.; Zou, J.; Drennan, J.; Miyayama, M. Diffusion and segregation along grain boundary at the electrolyte–anode interface in IT-SOFC. Solid State Ion. 2011, 191, 55–60. [Google Scholar] [CrossRef]
- Olyaeefar, B.; Ahmadi-Kandjani, S.; Asgari, A. Classical modelling of grain size and boundary effects in polycrystalline perovskite solar cells. Sol. Energy Mater. Sol. Cells 2018, 180, 76–82. [Google Scholar] [CrossRef]
- Nazem, H.; Dizaj, H.P.; Gorji, N.E. Modeling of Jsc and Voc versus the grain size in CdTe, CZTS and Perovskite thin film solar cells. Superlattices Microstruct. 2019, 128, 421–427. [Google Scholar] [CrossRef]
- Mastour, N.; Mejatty, M.; Bouchriha, H. Theoretical approach of the electroluminescence quenching in (polymer-CdSe quantum dot) nanocomposite. Superlattices Microstruct. 2015, 82, 461–471. [Google Scholar] [CrossRef]
- Korzhik, M.; Fedorov, A.; Dosovitskiy, G.; Anniyev, T.; Vasilyev, M.; Khabashesku, V. Nanoscale Engineering of Inorganic Composite Scintillation Materials. Materials 2021, 14, 4889. [Google Scholar] [CrossRef] [PubMed]
- Rudzik, T.J.; Seeley, Z.M.; Ryerson, F.J.; Cherepy, N.J.; Payne, S.A. Counter-ion effect on the diffusion behavior of Yb, Lu, and Nd ions in YAG transparent ceramics. Opt. Mater. X 2022, 13, 100132. [Google Scholar] [CrossRef]
- Fisher, J.C. Calculation of diffusion penetration curves for surface and grain boundary diffusion. J. Appl. Phys. 1951, 22, 74–77. [Google Scholar] [CrossRef]
- Whipple, R.T.P. Concentration contours in grain boundary diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 1225–1236. [Google Scholar] [CrossRef]
- Le Claire, A.D. The analysis of grain boundary diffusion measurements. Br. J. Appl. Phys. 1963, 14, 351–356. [Google Scholar] [CrossRef]
- Harrison, L.G. Influence of dislocations on diffusion kinetics in solids with particular reference to the alkali halides. Farad. Trans. 1961, 57, 1191–1199. [Google Scholar] [CrossRef]
- Divinski, S.V.; Lee, J.S.; Herzig, C. Grain Boundary Diffusion and Segregation in Compacted and Sintered Nanocrystalline Alloys. J. Metastable Nanocrystalline Mater. 2004, 19, 55–68. [Google Scholar] [CrossRef]
- Belova, I.V.; Fiedler, T.; Kulkarni, N.; Murch, G.E. The Harrison diffusion kinetics regimes in solute grain boundary diffusion. Philos. Mag. 2012, 92, 1748–1763. [Google Scholar] [CrossRef]
- Belova, I.V.; Murch, G.E. Investigation of Harrison Type-A, B and Intermediate AB Kinetics Regimes in Grain Boundary Diffusion. Defect Diffus. Forum. 2009, 283–286, 697–704. [Google Scholar] [CrossRef]
- Belova, I.V.; Murch, G.E.; Fiedler, T. Parametric Analysis of the Classification of Harrison Kinetics Regimes in Grain Boundary Diffusion. Defect Diffus. Forum. 2010, 297–301, 1226–1231. [Google Scholar] [CrossRef]
- Glienke, M.; Vaidya, M.; Guguraj, K.; Daum, L.; Tas, B.; Rogal, L.; Pradeep, K.G.; Divinski, S.V.; Wilde, G. Grain boundary diffusion in CoCrFeMnNi high entropy alloy: Kinetic hints towards a phase decomposition. Acta Mater. 2020, 195, 304–316. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Conforto, E.; Berziou, C.; Savall, C.; Feaugas, X. Grain size and grain-boundary effects on diffusion and trapping of hydrogen in pure nickel. Acta Mater. 2012, 60, 6814–6828. [Google Scholar] [CrossRef]
- Deng, J.; Ko, H.; Demkowitz, P.; Morgan, D.; Szlufarska, I. Grain boundary diffusion of Ag through polycrystalline SiC in TRISO fuel particles. J. Nucl. Mater. 2015, 467, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Chepak-Gizbrekht, M.V.; Knyazeva, A.G. Grain-boundary diffusion modeling in a microstructural material. Comput. Mater. Sci. 2020, 184, 109896. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Liu, G.; Li, C.; Kurosaka, S.; Nagao, S.; Suganuma, K. Enhancement of bonding strength in Ag sinter joining on Au surface finished substrate by increasing Au grain-size. Appl. Surf. Sci. 2019, 485, 468–475. [Google Scholar] [CrossRef]
- Lacaille, V.; Morel, C.; Feulvarch, E.; Kermouche, G.; Bergheau, J.M. Finite element analysis of the grain size effect on diffusion in polycrystalline materials. Comput. Mater. Sci. 2014, 95, 187–191. [Google Scholar] [CrossRef]
- Gryaznov, D.; Fleig, J.; Maier, J. Finite element simulation of diffusion into polycrystalline materials. Solid State Sci. 2008, 10, 754–760. [Google Scholar] [CrossRef]
- Zhang, J.; Fryauf, D.M.; Garrett, M.; Logeeswaran, V.J.; Sawabe, A.; Islam, M.S.; Kobayashi, N.P. Phenomenological model of the growth of ultrasmooth silver thin films deposited with a germanium nucleation layer. Langmuir 2015, 31, 7852–7859. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Skowronski, L.; Gorecka, E.; Kierdaszuk, J.; Szoplik, T. Growth model and structure evolution of Ag layers deposited on Ge films. Beilstein J. Nanotechnol. 2018, 9, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.S. Some elementary principles of polycrystalline microstructure. Metall. Rev. 1964, 9, 1–48. [Google Scholar] [CrossRef]
- Brockman, R.A.; Pilchak, A.L.; Porter III, W.J.; John, R. Estimation of grain boundary diffusivity in near-α titanium polycrystals. Scripta Mater. 2011, 65, 513–515. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.; Luo, Y.; He, Y.; Zhang, X.; Zhu, B.; Li, W.; Liu, X.; Ding, Y.; Li, Y.; et al. Advanced fuel cell based on new nanocrystalline structure Gd0. 1Ce0. 9O2 electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 10642–10650. [Google Scholar] [CrossRef]
- Iwaoka, H.; Arita, M.; Horita, Z. Hydrogen diffusion in ultrafine-grained palladium: Roles of dislocations and grain boundaries. Acta Mater. 2016, 107, 168–177. [Google Scholar] [CrossRef]
- Sowjanya, C.; Mandal, R.; Pratihar, S.K. Grain size dependent electrical conductivity, chemical surface exchange and bulk diffusion coefficient of La0.5Sr0.5Al0.2Fe0.8O3-δ. J. Alloy. Compd. 2020, 818, 152831. [Google Scholar] [CrossRef]
- Jiang, L.; Fu, C.T.; Leng, B.; Jia, Y.Y.; Ye, X.X.; Zhang, W.Z.; Bai, Q.; Xia, S.; Li, Z.J.; Liu, F.; et al. Influence of grain size on tellurium corrosion behaviors of GH3535 alloy. Corros. Sci. 2019, 148, 110–122. [Google Scholar] [CrossRef]
- Li, W.; Yu, W.; Xu, Q.; Zhou, J.; Nan, H.; Yin, Y.; Shen, X. Understanding the atomistic deformation mechanisms of polycrystalline γ-TiAl under nanoindentation: Effect of lamellar structure. J. Alloy. Compd. 2020, 828, 154443. [Google Scholar] [CrossRef]
- Edwards, T.E.J.; Di Gioacchino, F.; Clegg, W.J. An experimental study of the polycrystalline plasticity of lamellar titanium aluminide. Int. J. Plast. 2019, 118, 291–319. [Google Scholar] [CrossRef]
- Doan, D.Q.; Fang, T.H.; Chen, T.H. Effects of grain and twin boundary on friction and contact characteristics of CuZrAl nanocrystallines. Appl. Surf. Sci. 2020, 524, 146458. [Google Scholar] [CrossRef]
- Nagao, Y. Progress on highly proton-conductive polymer thin films with organized structure and molecularly oriented structure. Sci. Technol. Adv. Mater. 2020, 21, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Lütjering, G.E.R.D. Influence of processing on microstructure and mechanical properties of (α+ β) titanium alloys. Mater. Sci. Eng. A 1998, 243, 32–45. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Cheng, X.; Zhu, Y.; Zhang, J.; Wang, H. Effects of microstructure on fatigue crack propagation behavior in a bi-modal TC11 titanium alloy fabricated via laser additive manufacturing. J. Mater. Sci. Technol. 2019, 35, 403–408. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, W.; Shi, C.; Wang, H.; Jia, Z. The fracture toughness and its prediction model for Ti–5Al–5Mo–5V–1Cr–1Fe titanium alloy with basket-weave microstructure. J. Alloy. Compd. 2015, 632, 748–755. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, C.; Yan, Z.; Li, H.; Liu, Y. Formation mechanism and control methods of acicular ferrite in HSLA steels: A review. J. Mater. Sci. Technol. 2018, 34, 737–744. [Google Scholar] [CrossRef]
- Lapin, J.; Gebura, M.; Pelachova, T.; Nazmy, M. Coarsening kinetics of cuboidal γ’precipitates in single crystal nickel base superalloy CMSX-4. Kov. Mater. 2008, 46, 313–322. [Google Scholar]
- Wilson, B.C.; Hickman, J.A.; Fuchs, G.E. The effect of solution heat treatment on a single-crystal Ni-based superalloy. JOM 2003, 55, 35–40. [Google Scholar] [CrossRef]
- Birol, Y. Effect of solution heat treatment on the age hardening capacity of dendritic and globular AlSi7Mg0. 6 alloys. Int. J. Mater. Res. 2010, 101, 439–444. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhao, J.Z. Dendritic microstructure formation in a directionally solidified Al–11.6 Cu–0.85 Mg alloy. J. Cryst. Growth 2014, 391, 52–58. [Google Scholar] [CrossRef]
- Ares, A.E.; Gassa, L.M. Corrosion susceptibility of Zn–Al alloys with different grains and dendritic microstructures in NaCl solutions. Corros. Sci. 2012, 59, 290–306. [Google Scholar] [CrossRef]
- Li, C.; Li, J.C.; Zhao, M.; Jiang, Q. Effect of aluminum contents on microstructure and properties of AlxCoCrFeNi alloys. J. Alloy. Compd. 2010, 504, S515–S518. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, L.; Gao, L.; Guo, H.; Xu, H. Microstructural characterization of PS-PVD ceramic thermal barrier coatings with quasi-columnar structures. Surf. Coat. Technol. 2017, 311, 199–205. [Google Scholar] [CrossRef]
- Tiron, V. Tungsten nitride coatings obtained by HiPIMS as plasma facing materials for fusion applications. Appl. Surf. Sci. 2017, 416, 878–884. [Google Scholar] [CrossRef]
- Benedetti, I.; Barbe, F. Modelling polycrystalline materials: An overview of three-dimensional grain-scale mechanical models. J. Multiscale Model. 2013, 5, 1350002. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.C.; Choe, M.J.; Amatucci, G.G.; Chiang, Y.M.; Thornton, K. Smoothed boundary method for simulating bulk and grain boundary transport in complex polycrystalline microstructures. Comput. Mater. Sci. 2016, 121, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Naderi, S.; Dean, J.S.; Zhang, M. Three-dimensional virtual microstructure generation of porous polycrystalline ceramics. Ceram. Int. 2019, 45, 21647–21656. [Google Scholar] [CrossRef]
- Falco, S.; Jiang, J.; De Cola, F.; Petrinic, N. Generation of 3D polycrystalline microstructures with a conditioned Laguerre-Voronoi tessellation technique. Comput. Mater. Sci. 2017, 136, 20–28. [Google Scholar] [CrossRef]
- Romanova, V.; Balokhonov, R. A method of step-by-step packing and its application in generating 3D microstructures of polycrystalline and composite materials. Eng. Comput. 2021, 37, 241–250. [Google Scholar] [CrossRef]
- Jaseliūnaitė, J.; Galdikas, A. Kinetic modeling of grain boundary diffusion: The influence of grain size and surface processes. Materials 2020, 13, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannehill, J.C.; Anderson, D.A.; Pletcher, R.H. Computational Fluid Mechanics and Heat Transfer, 2nd ed.; Taylor & Francis: Washington, DC, USA, 1997; pp. 220–224. [Google Scholar]
- Holm, E.A.; Srolovitz, D.J.; Cahn, J.W. Microstructural evolution in two-dimensional two-phase polycrystals. Acta Metall. Et Mater. 1993, 41, 1119–1136. [Google Scholar] [CrossRef] [Green Version]












| Morphology | |||||
|---|---|---|---|---|---|
| Typical | 600,000 | 53,698 | 546,302 | 0.098 | 0.0741 |
| Bi-modal | 105,453 | 494,547 | 0.213 | 0.1182 | |
| Semi-lamellar | 135,484 | 464,516 | 0.292 | 0.1436 |
| Morphology | Diffusivity | Case 1 | Case 2 |
|---|---|---|---|
| Typical | Dgb | 0.1 | 0.1 |
| Dg | 0.0001 | 0.00001 | |
| Dgb/Dg | 1000 | 10000 | |
| Dtotal | 0.00750 | 0.00742 | |
| Bi-modal | Dgb | 0.1 | 0.1 |
| Dg | 0.0001 | 0.00001 | |
| Dgb/Dg | 1000 | 10000 | |
| Dtotal | 0.01191 | 0.01183 | |
| Semi-lamellar | Dgb | 0.1 | 0.1 |
| Dg | 0.0001 | 0.00001 | |
| Dgb/Dg | 1000 | 10000 | |
| Dtotal | 0.01445 | 0.01437 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaseliūnaitė, J.; Povilaitis, M.; Galdikas, A. Kinetic Modeling of Grain Boundary Diffusion: Typical, Bi-Modal, and Semi-Lamellar Polycrystalline Coating Morphologies. Coatings 2022, 12, 992. https://doi.org/10.3390/coatings12070992
Jaseliūnaitė J, Povilaitis M, Galdikas A. Kinetic Modeling of Grain Boundary Diffusion: Typical, Bi-Modal, and Semi-Lamellar Polycrystalline Coating Morphologies. Coatings. 2022; 12(7):992. https://doi.org/10.3390/coatings12070992
Chicago/Turabian StyleJaseliūnaitė, Justina, Mantas Povilaitis, and Arvaidas Galdikas. 2022. "Kinetic Modeling of Grain Boundary Diffusion: Typical, Bi-Modal, and Semi-Lamellar Polycrystalline Coating Morphologies" Coatings 12, no. 7: 992. https://doi.org/10.3390/coatings12070992