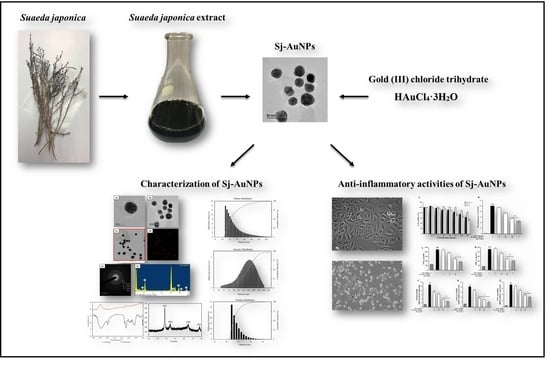
Gold Nanoparticles Green-Synthesized by the Suaeda japonica Leaf Extract and Screening of Anti-Inflammatory Activities on RAW 267.4 Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of an Aqueous Suaeda japonica Leaf Extract
2.3. Green Synthesis of Gold Nanoparticles Utilizing the Suaeda japonica Aqueous Extract
2.4. Characterization of Sj-AuNps
2.5. In Vitro Evaluation of the Cell Viability Assay
2.6. Measurement of NO, PGE2, and TNF-α Production
2.7. Gene Expression Studies
2.8. Immunofluorescence Staining
2.9. Statistical Analysis
3. Results and Discussion
3.1. Green Synthesis of Sj-AuNps
3.2. Characterizations of Sj-AuNps
3.3. In Vitro Applications of Sj-AuNps
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Coonoor, E.; Mwakmuka, J.; Gole, A. Gold nanoparticles are taken up by human cells but do not cause acute toxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold nanoparticles as potent anticancer agent: Green synthesis, characterization, and In Vitro study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Er, S.; Laraib, U.; Arshad, R.; Sargazi, S.; Rahdar, A.; Pandey, S.; Thakur, V.K.; Díez-Pascual, A.M. Amino acids, peptides, and proteins: Implications for nanotechnological applications in biosensing and drug/gene delivery. Nanomaterials 2021, 11, 3002. [Google Scholar] [CrossRef]
- Sharma, A.; Saini, A.K.; Kumar, N.; Tejwan, N.; Singh, T.A.; Thakur, V.K.; Das, J. Methods of preparation of metal-doped and hybrid tungsten oxide nanoparticles for anticancer, antibacterial, and biosensing applications. Surf. Interfaces 2022, 28, 101641. [Google Scholar] [CrossRef]
- Fatima, I.; Rahdar, A.; Sargazi, S.; Barani, M.; Hassanisaadi, M.; Thakur, V.K. Quantum Dots: Synthesis, Antibody Conjugation, and HER2-Receptor Targeting for Breast Cancer Therapy. J. Funct. Biomater. 2021, 12, 75. [Google Scholar] [CrossRef]
- Venditti, I. Engineered gold-based nanomaterials: Morphologies and functionalities in biomedical applications. a mini review. Bioengineering 2019, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Rajan, A.; Rajan, A.R.; Philip, D. Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. OpenNano 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Islam, N.U.; Jalil, K.; Shahid, M.; Muhammad, N.; Rauf, A. Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arab. J. Chem. 2019, 12, 2310–2319. [Google Scholar] [CrossRef]
- Szelenyi, I. Nanomedicine: Evolutionary and revolutionary developments in the treatment of certain inflammatory diseases. Inflamm. Res. 2012, 61, 1–9. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, G.H.S.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally Safe Biosynthesis of Gold Nanoparticles Using Plant Water Extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef]
- Silva, L.P.; Bonatto, C.C.; Polez, V.L.P. Green synthesis of metal nanoparticles by fungi: Current trends and challenges. In Advances and Applications through Fungal Nanobiotechnology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 71–89. [Google Scholar]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 1–26. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2020, 152, 104296. [Google Scholar] [CrossRef]
- Wang, B.; He, X.; Zhang, Z.; Zhao, Y.; Feng, W. Metabolism of nanomaterials in vivo: Blood circulation and organ clearance. Acc. Chem. Res. 2013, 46, 761–769. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Wee Yong, V. Inflammation in neurological disorders: A help or a hindrance? Neuroscientist 2010, 16, 408–420. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.-Y.; Lin, C.-F.; Aljuffali, I.A.; Fang, J.-Y. Specific targeting of engineered nanoparticles to activated macrophages. Curr. Nanosci. 2016, 12, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.-J.; Du, G.-H.; Qin, X.-M.; Gao, L. Baicalein attenuates the neuroinflammation in LPS-activated BV-2 microglial cells through suppression of pro-inflammatory cytokines, COX2/NF-κB expressions and regulation of metabolic abnormality. Int. Immunopharmacol. 2020, 79, 106092. [Google Scholar] [CrossRef]
- Jeong, J.B.; Jeong, H.J. Rheosmin, a naturally occurring phenolic compound inhibits LPS-induced iNOS and COX-2 expression in RAW264. 7 cells by blocking NF-κB activation pathway. Food Chem. Toxicol. 2010, 48, 2148–2153. [Google Scholar] [CrossRef]
- Wang, T.; Wu, F.; Jin, Z.; Zhai, Z.; Wang, Y.; Tu, B.; Yan, W.; Tang, T. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem. Toxicol. 2014, 64, 177–183. [Google Scholar] [CrossRef]
- Ahn, S.; Singh, P.; Castro-Aceituno, V.; Yesmin Simu, S.; Kim, Y.-J.; Mathiyalagan, R.; Yang, D.-C. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory-mediators production via blockade of NF-κB activation in macrophages. Artif. Cells Nanomed. Biotechnol. 2017, 45, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-Y.; Yang, X.; Park, K.-H.; Park, H.J.; Park, S.-Y.; Moon, J.-H.; Ham, K.-S. Isolation and identification of antioxidative compounds and their activities from Suaeda japonica. Food Sci. Biotechnol. 2013, 22, 1547–1557. [Google Scholar] [CrossRef]
- Choi, J.-I.; Kim, Y.-J.; Kim, J.-H.; Song, B.-S.; Yoon, Y.-H.; Byun, M.-W.; Kwon, J.-H.; Chun, S.-S.; Lee, J.-W. Antioxidant activities of the extract fractions from Suaeda japonica. J. Korean Soc. Food Sci Nutr. 2009, 38, 131–135. [Google Scholar] [CrossRef]
- Obón, C.; Rivera, D.; Verde, A.; Alcaraz, F. Ethnopharmacology and Medicinal Uses of Extreme Halophytes. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer Nature: Cham, Switzerland, 2020; pp. 1–29. [Google Scholar]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Markus, J.; Mathiyalagan, R.; Kim, Y.-J.; Abbai, R.; Singh, P.; Ahn, S.; Perez, Z.E.J.; Hurh, J.; Yang, D.C. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb. Technol. 2016, 95, 85–93. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.F.; Teng, Y.C.; Yoon, Y.S.; Kim, D.H.; Cai, D.Q.; Lee, K.J. Reactive oxygen species are involved in the IFN-γ-stimulated production of Th2 chemokines in HaCaT keratinocytes. J. Cell. Physiol. 2011, 226, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Chen, H.-L.; Li, H.; Zhang, K.-L.; Chen, X.-Y.; Wang, X.-W.; Kong, Q.-Y.; Liu, J. Regulatory effects of emodin on NF-κB activation and inflammatory cytokine expression in RAW 264.7 macrophages. Int. J. Mol. Med. 2005, 16, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Buceta, D.; Blanco-Varela, C.; Mohamed, M.B.; Barone, G.; López-Quintela, M.A. Structure-directing and high-efficiency photocatalytic hydrogen production by Ag clusters. J. Am. Chem. Soc. 2014, 136, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Markus, J.; Wang, D.; Kim, Y.-J.; Ahn, S.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of Chinese herbal Angelica pubescens Maxim. Nanoscale Res. Lett. 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geethalakshmi, R.; Sarada, D. Gold and silver nanoparticles from Trianthema decandra: Synthesis, characterization, and antimicrobial properties. Int. J. Nanomed. 2012, 7, 5375. [Google Scholar] [CrossRef] [Green Version]
- Borse, V.; Konwar, A.N. Synthesis and characterization of gold nanoparticles as a sensing tool for the lateral flow immunoassay development. Sens. Int. 2020, 1, 100051. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Wang, F.; Sun, C.; Wang, L.; Wang, J.; Sun, F. Enhancement of lipopolysaccharide-induced nitric oxide and interleukin-6 production by PEGylated gold nanoparticles in RAW264.7 cells. Nanoscale 2012, 4, 7135–7142. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-kB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Mei, H.; Wen, J.K.; Zheng, B.; Zhang, D.Q. Acetylbritannilatone suppresses NO and PGE2 synthesis in RAW 264.7 macrophages through the inhibition of iNOS and COX-2 gene expression. Life Sci. 2004, 75, 675–684. [Google Scholar]
- Lee, J.M.; Yim, M.-J.; Choi, G.; Lee, M.S.; Park, Y.G.; Lee, D.-S. Antioxidant and anti-inflammatory activity of six halophytes in Korea. Nat. Prod. Sci. 2018, 24, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Pasparakis, M.; Alexopoulou, L.; Episkopou, V.; Kollias, G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996, 184, 1397–1411. [Google Scholar] [CrossRef]
- Waetzig, G.H.; Seegert, D.; Rosenstiel, P.; Nikolaus, S.; Schreiber, S. p38 Mitogen-Activated Protein Kinase Is Activated and Linked to TNF-α Signaling in Inflammatory Bowel Disease. J. Immunol. 2002, 168, 5342–5351. [Google Scholar] [CrossRef] [Green Version]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.L.; Kumar, P.; Sharma, S.; Dhiman, K.; Verma, A. Gold Nanoparticle-Mediated Delivery of Therapeutic Enzymes for Biomedical Applications. Nanosci. Med. 2020, 1, 89–115. [Google Scholar]








| Primer Name | Sequence | Tm (°C) |
|---|---|---|
| iNOS | Forward: 5′-GTG GTG ACA ACG ACA TTT GG-3′ | 57.3 |
| Reverse: 5′-GGC TGG ACT TTT CAC TCT GC-3′ | 59.3 | |
| COX-2 | Forward: 5′-GGA TGC GCT GAA ACG TGG A-3′ | 58.8 |
| Reverse: 5′-CAG GAA TGA GTA CAC GAA GCC-3′ | 59.8 | |
| TNF-α | Forward: 5′-AGT CCG GGC AGG TCT ACT TT-3′ | 59.3 |
| Reverse: 5′-GCA CCT CAG GGA AGA GTC TG-3′ | 61.4 | |
| GAPDH | Forward: 5′-CAA GGT CAT CCA TGA CAA CTT TG-3′ | 59.4 |
| Reverse: 5′-GTC CAC CAC CCT GTT GCT GTA G-3′ | 64.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, G.-Y.; Han, Y.; Baik, S.; Kong, B.-M.; Yang, D.-C.; Kang, S.-C.; Sukweenadhi, J. Gold Nanoparticles Green-Synthesized by the Suaeda japonica Leaf Extract and Screening of Anti-Inflammatory Activities on RAW 267.4 Macrophages. Coatings 2022, 12, 460. https://doi.org/10.3390/coatings12040460
Kwak G-Y, Han Y, Baik S, Kong B-M, Yang D-C, Kang S-C, Sukweenadhi J. Gold Nanoparticles Green-Synthesized by the Suaeda japonica Leaf Extract and Screening of Anti-Inflammatory Activities on RAW 267.4 Macrophages. Coatings. 2022; 12(4):460. https://doi.org/10.3390/coatings12040460
Chicago/Turabian StyleKwak, Gi-Young, Yaxi Han, Sul Baik, Byoung-Man Kong, Deok-Chun Yang, Se-Chan Kang, and Johan Sukweenadhi. 2022. "Gold Nanoparticles Green-Synthesized by the Suaeda japonica Leaf Extract and Screening of Anti-Inflammatory Activities on RAW 267.4 Macrophages" Coatings 12, no. 4: 460. https://doi.org/10.3390/coatings12040460