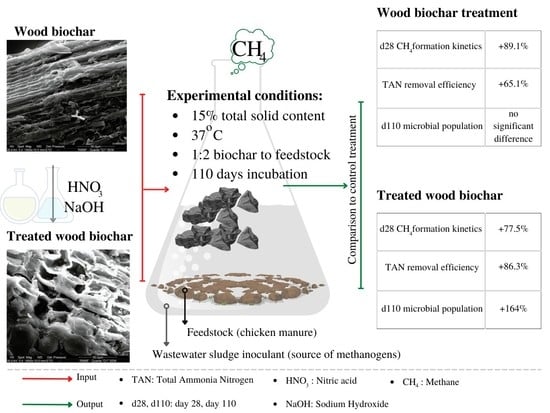
Wood Biochar Enhances the Valorisation of the Anaerobic Digestion of Chicken Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anaerobic Digestion Feedstock and Inoculant Characterisation
2.2. Biochar Preparation and Characterisation
2.3. Anaerobic Digestion Set-up and Biogas Sampling
2.4. Methane-Production Determination
2.5. Post-Digestion Chemical Analysis
2.6. DNA Extraction
2.7. Real-Time Quantitative Polymerase Chain Reaction
2.8. Data Analysis
3. Results and Discussions
3.1. Effects of Biochar (BC) and Treated-Biochar (TBC) Treatments on Biomethane Production
3.2. Total Ammonia Nitrogen (TAN) Removal Efficiency
3.3. Digestate Characteristics
3.3.1. Chemical Oxygen Demand (COD)
3.3.2. Electrical Conductivity (EC)
3.3.3. Salinity
3.3.4. pH
3.4. Changes in Microbial Biomass during Anaerobic Digestion
3.5. Direct Interspecies Electron Transfer
3.6. Biochar Characteristics
3.6.1. Biochar Surface Morphology
3.6.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.6.3. X-ray Fluorescence (XRF) Analysis
3.6.4. Fourier Transformed Infrared (FTIR) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, P.A.; Daniel, T.C.; Sharpley, A.N.; Wood, C.W. Poultry Manure Management; United States Department of Agriculture, Ed.; United States Department of Agriculture: Washington, DC, USA, 2021.
- Jurgutis, L.; Slepetiene, A.; Volungevicius, J.; Amaleviciute-Volunge, K. Biogas production from chicken manure at different organic loading rates in a mesophilic full scale anaerobic digestion plant. Biomass Bioenergy 2020, 141, 105693. [Google Scholar] [CrossRef]
- Chen, B.; Shao, Y.; Shi, M.; Ji, L.; He, Q.; Yan, S. Anaerobic digestion of chicken manure coupled with ammonia recovery by vacuum-assisted gas-permeable membrane process. Biochem. Eng. J. 2021, 175, 108135. [Google Scholar] [CrossRef]
- Junga, R.; Knauer, W.; Niemiec, P.; Tańczuk, M. Experimental tests of co-combustion of laying hens manure with coal by using thermogravimetric analysis. Renew. Energy 2017, 111, 245–255. [Google Scholar] [CrossRef]
- Ngo, T.; Shahsavari, E.; Shah, K.; Surapaneni, A.; Ball, A.S. Improving bioenergy production in anaerobic digestion systems utilising chicken manure via pyrolysed biochar additives: A review. Fuel 2022, 316, 123374. [Google Scholar] [CrossRef]
- Shi, X.; Lin, J.; Zuo, J.; Li, P.; Li, X.; Guo, X. Effects of free ammonia on volatile fatty acid accumulation and process performance in the anaerobic digestion of two typical bio-wastes. J. Environ. Sci. 2017, 55, 49–57. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Puig-Castellví, F.; Cardona, L.; Bureau, C.; Bouveresse, D.J.-R.; Cordella, C.B.; Mazéas, L.; Rutledge, D.N.; Chapleur, O. Effect of ammonia exposure and acclimation on the performance and the microbiome of anaerobic digestion. Bioresour. Technol. Rep. 2020, 11, 100488. [Google Scholar] [CrossRef]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour. Technol. 2021, 325, 124697. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Zhang, D.-L.; Yi, W.-M.; Liu, Y. Effects of acid modification on the structure and adsorption NH4+-N properties of biochar. Renew. Energy 2021, 169, 1343–1350. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Ye, Z.-L.; Chen, S. Engineered biochars for recovering phosphate and ammonium from wastewater: A review. Sci. Total Environ. 2021, 779, 146240. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chu, Y.; Zhu, J.; Sheng, L.; Liu, G.; Xing, Y.; Fu, P.; Li, Q.; Chen, R. Multi-faceted influences of biochar addition on swine manure digestion under tetracycline antibiotic pressure. Bioresour. Technol. 2022, 346, 126352. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhang, R.; Zhao, R. Adsorption characters of ammonium-nitrogen in aqueous solution by modified corn cob biochars. CIESC J. 2014, 65, 960–966. [Google Scholar]
- Surendra, K.; Takara, D.; Hashimoto, A.G.; Khanal, S.K. Biogas as a sustainable energy source for developing countries: Opportunities and challenges. Renew. Sustain. Energy Rev. 2014, 31, 846–859. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.; Khan, E.; Yip, A.C.; Ng, Y.H.; Rinklebe, J.; Ok, Y.S. Customised fabrication of nitrogen-doped biochar for environmental and energy applications. Chem. Eng. J. 2020, 401, 126136. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Kang, X.; Wu, B.; Wall, D.M.; Murphy, J.D. What physicochemical properties of biochar facilitate interspecies electron transfer in anaerobic digestion: A case study of digestion of whiskey by-products. Fuel 2021, 306, 121736. [Google Scholar] [CrossRef]
- Lian, F.; Cui, G.; Liu, Z.; Duo, L.; Zhang, G.; Xing, B. One-step synthesis of a novel N-doped microporous biochar derived from crop straws with high dye adsorption capacity. J. Environ. Manag. 2016, 176, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Bi, S.; Westerholm, M.; Qiao, W.; Mahdy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced methanogenic performance and metabolic pathway of high solid anaerobic digestion of chicken manure by Fe2+ and Ni2+ supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ryan, D.; Murphy, R.; Enright, A.M. A comprehensive review of green policy, anaerobic digestion of animal manure and chicken litter feedstock potential—Global and Irish perspective. Renew. Sustain. Energy Rev. 2022, 154, 111884. [Google Scholar] [CrossRef]
- Indren, M.; Birzer, C.; Kidd, S.; Medwell, P.R. Effect of total solids content on anaerobic digestion of poultry litter with biochar. J. Environ. Manag. 2020, 255, 109744. [Google Scholar] [CrossRef] [PubMed]
- Indren, M.; Birzer, C.H.; Kidd, S.P.; Hall, T.; Medwell, P.R. Effect of wood biochar dosage and re-use on high-solids anaerobic digestion of chicken litter. Biomass Bioenergy 2021, 144, 105872. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kassongo, J.; Shahsavari, E.; Ball, A.S. Renewable energy from the solid-state anaerobic digestion of grape marc and cheese whey at high treatment capacity. Biomass Bioenergy 2020, 143, 105880. [Google Scholar] [CrossRef]
- Gao, B.; Wang, Y.; Huang, L.; Liu, S. Study on the performance of HNO3-modified biochar for enhanced medium temperature anaerobic digestion of food waste. Waste Manag. 2021, 135, 338–346. [Google Scholar] [CrossRef]
- Kassongo, J.; Shahsavari, E.; Ball, A.S. Dynamic Effect of Operational Regulation on the Mesophilic BioMethanation of Grape Marc. Molecules 2021, 26, 6692. [Google Scholar] [CrossRef]
- IEA Bioenergy. Mono-Digestion of Chicken Litter: Tully Biogas Plant, Ballymena, Northern Ireland. 2019. Available online: https://www.ieabioenergy.com/blog/publications/mono-digestion-of-chicken-litter-tully-biogas-plant-ballymena-northern-ireland/ (accessed on 15 April 2022).
- Shahsavari, E.; Aburto-Medina, A.; Taha, M.; Ball, A.S. A quantitative PCR approach for quantification of functional genes involved in the degradation of polycyclic aromatic hydrocarbons in contaminated soils. MethodsX 2016, 3, 205–211. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, C.; Liu, R.; Yellezuome, D.; Zhu, X.; Bai, R.; Liu, M.; Sun, M. Anaerobic co-digestion of corn stover and chicken manure using continuous stirred tank reactor: The effect of biochar addition and urea pretreatment. Bioresour. Technol. 2021, 319, 124197. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Watson, J.; Sharma, B.K.; Si, B.; Zhang, Y. Adsorption or direct interspecies electron transfer? A comprehensive investigation of the role of biochar in anaerobic digestion of hydrothermal liquefaction aqueous phase. Chem. Eng. J. 2022, 435, 135078. [Google Scholar] [CrossRef]
- Bonk, F.; Popp, D.; Weinrich, S.; Sträuber, H.; Kleinsteuber, S.; Harms, H.; Centler, F. Ammonia Inhibition of Anaerobic Volatile Fatty Acid Degrading Microbial Communities. Front. Microbiol. 2018, 9, 2921. [Google Scholar] [CrossRef] [PubMed]
- Waltham, B.; Örmeci, B. Fluorescence intensity, conductivity, and UV–vis absorbance as surrogate parameters for real-time monitoring of anaerobic digestion of wastewater sludge. J. Water Process Eng. 2020, 37, 101395. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; He, Q.; Ji, B.; Wang, H.; Yang, K. Adaptation to salinity: Response of biogas production and microbial communities in anaerobic digestion of kitchen waste to salinity stress. J. Biosci. Bioeng. 2020, 130, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Kovalovszki, A.; Alvarado-Morales, M.; Rudatis, A.; Kougias, P.G.; Angelidaki, I. Anaerobic co-digestion of macroalgal biomass with cattle manure under high salinity conditions. J. Environ. Chem. Eng. 2021, 9, 105406. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Lü, F.; Liu, Y.; Shao, L.; He, P. Powdered biochar doubled microbial growth in anaerobic digestion of oil. Appl. Energy 2019, 247, 605–614. [Google Scholar] [CrossRef]
- Madigou, C.; Poirier, S.; Bureau, C.; Chapleur, O. Acclimation strategy to increase phenol tolerance of an anaerobic microbiota. Bioresour. Technol. 2016, 216, 77–86. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Yan, M.; Ravenni, G.; Angelidaki, I.; Fu, D.; Fotidis, I.A. Novel bioaugmentation strategy boosted with biochar to alleviate ammonia toxicity in continuous biomethanation. Bioresour. Technol. 2022, 343, 126146. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Wu, J.; Li, Y.; Ali, N.; Ding, S.; Wang, K. Effect of biochar on reactor performance and methane generation during the anaerobic digestion of food waste treatment at long-run operations. J. Environ. Chem. Eng. 2019, 7, 103067. [Google Scholar] [CrossRef]
- Gahlot, P.; Ahmed, B.; Tiwari, S.B.; Aryal, N.; Khursheed, A.; Kazmi, A.; Tyagi, V.K. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Beckmann, S.; Welte, C.; Li, X.; Oo, Y.M.; Kroeninger, L.; Heo, Y.; Zhang, M.; Ribeiro, D.; Lee, M.; Bhadbhade, M.; et al. Novel phenazine crystals enable direct electron transfer to methanogens in anaerobic digestion by redox potential modulation. Energy Environ. Sci. 2016, 9, 644–655. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, Q.; Li, Y.; Xing, Y.; Yao, G.; Liu, Y.; Chen, R.; Wang, X. Redox-active biochar facilitates potential electron tranfer between syntrophic partners to enhance anaerobic digestion under high organic loading rate. Bioresour. Technol. 2020, 298, 122524. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhu, M.; Meng, X.; Zhou, J.L.; Zhang, H.; Shen, X. The role of biochar on alleviating ammonia toxicity in anaerobic digestion of nitrogen-rich wastes: A review. Bioresour. Technol. 2022, 351, 126924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, J.; Saleem, M.; Yang, Y.; Zhang, Q. Enhanced adsorptive removal of carbendazim from water by FeCl3-modified corn straw biochar as compared with pristine, HCl and NaOH modification. J. Environ. Chem. Eng. 2022, 10, 107024. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Zhao, L.; Li, Z.; Yi, X.; Guo, T.; Cao, X. Enhanced trichloroethylene biodegradation: Roles of biochar-microbial collaboration beyond adsorption. Sci. Total Environ. 2021, 792, 148451. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Li, A.-Y.; Deng, H.; Ye, C.-H.; Wu, Y.-Q.; Linmu, Y.-D.; Hang, H.-L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-H.; Huang, Y.-Y. Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J. Clean. Prod. 2018, 175, 354–360. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Lu, B.; Wang, X.; Huang, H. LaMnO3 nanoparticles supported on N doped porous carbon as efficient photocatalyst. Vacuum 2019, 159, 59–68. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Rahimian, P.; Kaghazchi, T. Modified activated carbons with amino groups and their copper adsorption properties in aqueous solution. Chin. J. Chem. Eng. 2015, 23, 50–56. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Wang, S.; Ai, S.; Nzediegwu, C.; Kwak, J.-H.; Islam, S.; Li, Y.; Chang, S.X. Carboxyl and hydroxyl groups enhance ammonium adsorption capacity of iron (III) chloride and hydrochloric acid modified biochars. Bioresour. Technol. 2020, 309, 123390. [Google Scholar] [CrossRef]
- Munar-Florez, D.A.; Varón-Cardenas, D.A.; Ramírez-Contreras, N.E.; García-Núñez, J.A. Adsorption of ammonium and phosphates by biochar produced from oil palm shells: Effects of production conditions. Results Chem. 2021, 3, 100119. [Google Scholar] [CrossRef]
- Zhang, M.; Shu, L.; Guo, X.; Shen, X.; Zhang, H.; Shen, G.; Wang, B.; Yang, Y.; Tao, S.; Wang, X. Impact of humic acid coating on sorption of naphthalene by biochars. Carbon 2015, 94, 946–954. [Google Scholar] [CrossRef]
- Han, L.; Wu, W.; Chen, X.; Chen, M. Co-sorption/co-desorption mechanism of the mixed chlorobenzenes by fresh bulk and aged residual biochar. J. Hazard. Mater. 2022, 429, 128349. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z.; Skic, K.; Tomczyk, A. Chemically engineered biochar—Effect of concentration and type of modifier on sorption and structural properties of biochar from wood waste. Fuel 2019, 256, 115893. [Google Scholar] [CrossRef]
- Yang, G.-X.; Jiang, H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Fang, Y.; Lai, W.; Xu, S.; Lichtfouse, E. Enhancing thermophilic anaerobic co-digestion of sewage sludge and food waste with biogas residue biochar. Renew. Energy 2022, 188, 465–475. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; Xue, Y. Nickel-loaded shrimp shell biochar enhances batch anaerobic digestion of food waste. Bioresour. Technol. 2022, 352, 127092. [Google Scholar] [CrossRef]





| Properties | Unit | Chicken Manure | Inoculant | Chicken Manure and Inoculant |
|---|---|---|---|---|
| Total chemical oxygen demand (CODt) | g L−1 | 14.4 ± 0.40 | 2.48 ± 0.07 | 12.6 ± 0.31 |
| Soluble chemical oxygen demand (CODs) | g L−1 | 13.5 ± 0.85 | 2.14 ± 0.16 | 7.78 ± 0.17 |
| Volatile solids (VSs) | % | 59.7 ± 3.31 | 1.89 ± 0.43 | 11.9 ± 0.53 |
| Total solids (TSs) | % | 80.9 ± 0.36 | 2.67 ± 0.03 | 14.4 ± 0.38 |
| Electrical conductivity (EC) | mS cm−1 | 52.6 ± 0.17 | 3.82 ± 0.09 | 25.8 ± 0.26 |
| Salinity | % | 23.0 ± 1.73 | 2.33 ± 0.58 | 11.3 ± 0.57 |
| pH | - | 8.61 ± 0.01 | 7.49 ± 0.02 | 7.41 ± 0.01 |
| Total ammonia nitrogen (TAN) | mg L−1 | 2600 ± 0.00 | 200 ± 0.00 | 2267 ± 46.2 |
| Treatment | d0 TAN (mg-TAN l−1) | d110 TAN (mg-TAN l−1) | Increase in TAN (mg-TAN l−1) | Increase in TAN (%) | TAN Removal Efficiency (%) |
|---|---|---|---|---|---|
|
Control (No biochar) | 2267 | 3822 | 1556 | 68.6 | 0 |
|
Wood biochar (BC) | 2533 | 3075 | 542 | 21.4 | 65.1 |
|
Treated biochar (TBC) | 2560 | 2773 | 213 | 8.33 | 86.3 |
| Parameters | Chicken Manure and Inoculant (No Biochar) | Chicken Manure and Inoculant + Biochar (BC Treatment) | Chicken Manure and Inoculant+ Treated Biochar (TBC Treatment) | |||
|---|---|---|---|---|---|---|
| Value | % Change | Value | % Change | Value | % Change | |
| Total chemical oxygen demand (g L−1) | 3.71 ± 0.19 | −70.4 | 3.6 ± 0.25 | −71.4 | 2.98 ± 0.3 | −76.2 |
| Soluble chemical oxygen demand (g L−1) | 2.18 ± 0.11 | −71.9 | 2.1 ± 0.11 | −72.9 | 1.77 ± 0.11 | −77.0 |
|
Electrical conductivity (mS cm−1) | 32.0 ± 1.73 | +24.1 | 26.2 ± 0.95 | +1.55 | 31.4 ± 1.75 | +21.7 |
| Salinity (%) | 15 ± 0.87 | +32.4 | 12.4 ± 0.53 | +9.4 | 15.6 ± 0.53 | +37.6 |
| pH | 8.31 ± 0.01 | N/A | 8.30 ± 0.05 | N/A | 8.41 ± 0.08 | N/A |
| Analytical Method | Characteristics/Units | BC | TBC | |
|---|---|---|---|---|
| pH | 7.93 | 8.47 | ||
| X-Ray photoelectron spectroscopy | Surface composition (%) | C | 80.38 | 74.10 |
| O | 18.90 | 20.79 | ||
| N | 0.71 | 2.90 | ||
| Na | 0.00 | 2.21 | ||
| Carbon bonding (%) | C-C/C-H | 31.95 | 40.22 | |
| C-O | 44.49 | 27.9 | ||
| C-OOH | 3.94 | 5.98 | ||
| Nitrogen bonding (%) | Pyrrolic N | 0.71 | 1.34 | |
| Nitrate (NO3−) | 0.00 | 1.56 | ||
| Cation exchange capacity | meq/100 g | 17 | 31 | |
| X-ray fluorescence | Metal oxide composition (%) | Na2O | 0.00 | 3.09 |
| CaO | 10.91 | 2.59 | ||
| MgO | 0.18 | 0.07 | ||
| Al2O3 | 0.16 | 0.04 | ||
| SiO2 | 0.62 | 0.25 | ||
| Fe2O3 | 0.59 | 0.34 | ||
| K2O | 1.93 | 0.31 | ||
| Waveband (cm−1) | Functional Groups |
|---|---|
| 3500–3250 | -OH groups in oximes |
| 2834–3100 | -OH groups in carboxylic acids |
| 1670–1543 | -NH2 groups |
| 1543–1470 | -NH, NO2 groups |
| 1470–1290 | COOH stretching |
| 1400–1440 | -OH groups |
| 1290–1160 | C-O-C (esters) bond |
| 1200–1000 | C-OH group in alcohols |
| 1043–922 | C-C aliphatic bond |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, T.; Khudur, L.S.; Hakeem, I.G.; Shah, K.; Surapaneni, A.; Ball, A.S. Wood Biochar Enhances the Valorisation of the Anaerobic Digestion of Chicken Manure. Clean Technol. 2022, 4, 420-439. https://doi.org/10.3390/cleantechnol4020026
Ngo T, Khudur LS, Hakeem IG, Shah K, Surapaneni A, Ball AS. Wood Biochar Enhances the Valorisation of the Anaerobic Digestion of Chicken Manure. Clean Technologies. 2022; 4(2):420-439. https://doi.org/10.3390/cleantechnol4020026
Chicago/Turabian StyleNgo, Tien, Leadin S. Khudur, Ibrahim Gbolahan Hakeem, Kalpit Shah, Aravind Surapaneni, and Andrew S. Ball. 2022. "Wood Biochar Enhances the Valorisation of the Anaerobic Digestion of Chicken Manure" Clean Technologies 4, no. 2: 420-439. https://doi.org/10.3390/cleantechnol4020026