Approach to the Connection between Meconium Consistency and Adverse Neonatal Outcomes: A Retrospective Clinical Review and Prospective In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Study
Data Sources
2.2. Cell Study
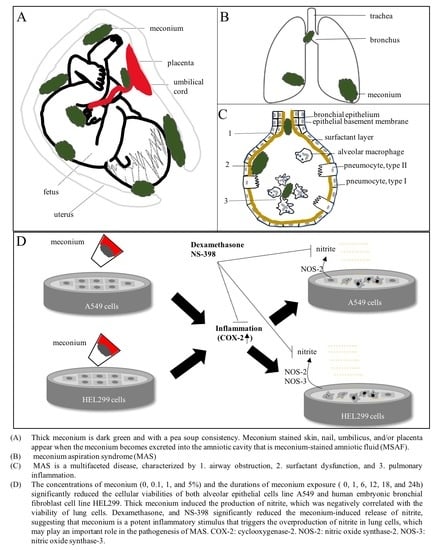
2.2.1. Preparation of Meconium
2.2.2. Culture of Lung Cells
2.2.3. Meconium Stimulation
2.2.4. Cell Viability
2.2.5. Nitrite Determination
2.2.6. RNA Extraction and Real-Time Quantitative PCR
2.2.7. Library Preparation and Sequencing
2.2.8. Bioinformatics
2.2.9. Statistical Analysis
3. Results
3.1. Thick Meconium Is a Risk Factor for Neonates Receiving Resuscitation
3.2. Thick Meconium with Longer Exposure Times Induces Lung Cell Death
3.3. Meconium Induces NOS and COX Gene Expression
3.4. Meconium Enhances Nitrite Production
3.5. Dexamethasone and COX-2 Inhibitor Treatment Significantly Reduced the Nitrite Production Induced by Meconium Stimulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonowicz, I.; Shwachman, H. Meconium in health and in disease. Adv. Pediatr. 1979, 26, 275–310. [Google Scholar] [PubMed]
- Holtzman, R.B.; Banzhaf, W.C.; Silver, R.K.; Hageman, J.R. Perinatal management of meconium staining of the amniotic fluid. Clin. Perinatol. 1989, 16, 825–838. [Google Scholar] [CrossRef]
- Romero, R.; Yoon, B.H.; Chaemsaithong, P.; Cortez, J.; Park, C.W.; Gonzalez, R.; Behnke, E.; Hassan, S.S.; Chaiworapongsa, T.; Yeo, L. Bacteria and endotoxin in meconium-stained amniotic fluid at term: Could intra-amniotic infection cause meconium passage? J. Matern. Fetal Neonatal Med. 2014, 27, 775–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.; Unsworth, J.; Vause, S. Meconium in labour. Obstet. Gynaecol. Reprod. Med. 2013, 23, 247–252. [Google Scholar] [CrossRef]
- Romero, R.; Yoon, B.H.; Chaemsaithong, P.; Cortez, J.; Park, C.W.; Gonzalez, R.; Behnke, E.; Hassan, S.S.; Gotsch, F.; Yeo, L.; et al. Secreted phospholipase a2 is increased in meconium-stained amniotic fluid of term gestations: Potential implications for the genesis of meconium aspiration syndrome. J. Matern. Fetal Neonatal Med. 2014, 27, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Ahanya, S.N.; Lakshmanan, J.; Morgan, B.L.; Ross, M.G. Meconium passage in utero: Mechanisms, consequences, and management. Obstet. Gynecol. Surv. 2005, 60, 45–56. [Google Scholar] [CrossRef]
- Dargaville, P.A.; Copnell, B.; Australian and New Zealand Neonatal Network. The epidemiology of meconium aspiration syndrome: Incidence, risk factors, therapies, and outcome. Pediatrics 2006, 117, 1712–1721. [Google Scholar] [CrossRef]
- van Ierland, Y.; de Beaufort, A.J. Why does meconium cause meconium aspiration syndrome? Current concepts of mas pathophysiology. Early Hum. Dev. 2009, 85, 617–620. [Google Scholar] [CrossRef]
- Zagariya, A.; Bhat, R.; Navale, S.; Vidyasagar, D. Cytokine expression in meconium-induced lungs. Indian J. Pediatr. 2004, 71, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ziadeh, S.M.; Sunna, E. Obstetric and perinatal outcome of pregnancies with term labour and meconium-stained amniotic fluid. Arch. Gynecol. Obstet. 2000, 264, 84–87. [Google Scholar] [CrossRef]
- Poggi, S.H.; Ghidini, A. Pathophysiology of meconium passage into the amniotic fluid. Early Hum. Dev. 2009, 85, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, A.O.; Tanyel, F.C.; Karnak, I.; Buyukpamukcu, N.; Hicsonmez, A. In-utero defecation: Fact or fiction? Eur. J. Pediatr. Surg. 1999, 9, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Monen, L.; Hasaart, T.H.; Kuppens, S.M. The aetiology of meconium-stained amniotic fluid: Pathologic hypoxia or physiologic foetal ripening? Early Hum. Dev. 2014, 90, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Fanaroff, J.M. Meconium stained fluid: Approach to the mother and the baby. Clin. Perinatol. 2007, 34, 653–665. [Google Scholar] [CrossRef]
- Westgate, J.A.; Bennet, L.; Gunn, A.J. Meconium and fetal hypoxia: Some experimental observations and clinical relevance. BJOG 2002, 109, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.J.; Ross, M.G.; Day, L.; Humme, J.; Ervin, M.G. Fetal swallowing: Response to graded maternal hypoxemia. J. Appl. Physiol. 1991, 71, 1856–1861. [Google Scholar] [CrossRef]
- Ohana, O.; Holcberg, G.; Sergienko, R.; Sheiner, E. Risk factors for intrauterine fetal death (1988–2009). J. Matern. Fetal Neonatal Med. 2011, 24, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Brailovschi, Y.; Sheiner, E.; Wiznitzer, A.; Shahaf, P.; Levy, A. Risk factors for intrapartum fetal death and trends over the years. Arch. Gynecol. Obstet. 2012, 285, 323–329. [Google Scholar] [CrossRef]
- Kalis, V.; Turek, J.; Hudec, A.; Rokyta, P.; Rokyta, Z.; Mejchar, B. Meconium and postnatal neurologic handicaps. Ceska Gynekol. 2001, 66, 369–377. [Google Scholar]
- Redline, R.W. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am. J. Obstet. Gynecol. 2005, 192, 452–457. [Google Scholar] [CrossRef]
- Sheiner, E.; Hadar, A.; Shoham-Vardi, I.; Hallak, M.; Katz, M.; Mazor, M. The effect of meconium on perinatal outcome: A prospective analysis. J. Matern. Fetal Neonatal Med. 2002, 11, 54–59. [Google Scholar] [CrossRef]
- Fischer, C.; Rybakowski, C.; Ferdynus, C.; Sagot, P.; Gouyon, J.B. A population-based study of meconium aspiration syndrome in neonates born between 37 and 43 weeks of gestation. Int. J. Pediatr. 2012, 2012, 321545. [Google Scholar] [CrossRef]
- Gupta, V.; Bhatia, B.D.; Mishra, O.P. Meconium stained amniotic fluid: Antenatal, intrapartum and neonatal attributes. Indian Pediatr. 1996, 33, 293–297. [Google Scholar]
- Monfredini, C.; Cavallin, F.; Villani, P.E.; Paterlini, G.; Allais, B.; Trevisanuto, D. Meconium aspiration syndrome: A narrative review. Children 2021, 8, 230. [Google Scholar] [CrossRef]
- Bhutani, V.K. Developing a systems approach to prevent meconium aspiration syndrome: Lessons learned from multinational studies. J. Perinatol. 2008, 28 (Suppl. S3), S30–S35. [Google Scholar] [CrossRef] [Green Version]
- Olicker, A.L.; Raffay, T.M.; Ryan, R.M. Neonatal respiratory distress secondary to meconium aspiration syndrome. Children 2021, 8, 246. [Google Scholar] [CrossRef]
- Beligere, N.; Rao, R. Neurodevelopmental outcome of infants with meconium aspiration syndrome: Report of a study and literature review. J. Perinatol. 2008, 28 (Suppl. S3), S93–S101. [Google Scholar] [CrossRef] [Green Version]
- Naeye, R.L. Can meconium in the amniotic fluid injure the fetal brain? Obstet. Gynecol. 1995, 86, 720–724. [Google Scholar] [CrossRef]
- Thornton, P.D.; Campbell, R.T.; Mogos, M.F.; Klima, C.S.; Parsson, J.; Strid, M. Meconium aspiration syndrome: Incidence and outcomes using discharge data. Early Hum. Dev. 2019, 136, 21–26. [Google Scholar] [CrossRef]
- Rawat, M.; Nangia, S.; Chandrasekharan, P.; Lakshminrusimha, S. Approach to infants born through meconium stained amniotic fluid: Evolution based on evidence? Am. J. Perinatol. 2018, 35, 815–822. [Google Scholar]
- Perlman, J.M.; Wyllie, J.; Kattwinkel, J.; Atkins, D.L.; Chameides, L.; Goldsmith, J.P.; Guinsburg, R.; Hazinski, M.F.; Morley, C.; Richmond, S.; et al. Part 11: Neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2010, 122, S516–S538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyllie, J.; Perlman, J.M.; Kattwinkel, J.; Atkins, D.L.; Chameides, L.; Goldsmith, J.P.; Guinsburg, R.; Hazinski, M.F.; Morley, C.; Richmond, S.; et al. Part 11: Neonatal resuscitation: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2010, 81 (Suppl. S1), e260–e287. [Google Scholar] [CrossRef] [PubMed]
- Kopincova, J.; Calkovska, A. Meconium-induced inflammation and surfactant inactivation: Specifics of molecular mechanisms. Pediatr. Res. 2016, 79, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rodriguez, E.; Perez-Gil, J. Structure-function relationships in pulmonary surfactant membranes: From biophysics to therapy. Biochim. Biophys. Acta 2014, 1838, 1568–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikolka, P.; Kopincova, J.; Tomcikova Mikusiakova, L.; Kosutova, P.; Antosova, M.; Calkovska, A.; Mokra, D. Effects of surfactant/budesonide therapy on oxidative modifications in the lung in experimental meconium-induced lung injury. J. Physiol. Pharmacol. 2016, 67, 57–65. [Google Scholar] [PubMed]
- El Shahed, A.I.; Dargaville, P.A.; Ohlsson, A.; Soll, R. Surfactant for meconium aspiration syndrome in term and late preterm infants. Cochrane Database Syst. Rev. 2014, 2014, CD002054. [Google Scholar] [CrossRef]
- Polin, R.A.; Carlo, W.A.; Committee on Fetus and Newborn. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Am. Acad. Pediatr. 2014, 133, 156–163. [Google Scholar]
- Vidyasagar, D.; Lukkarinen, H.; Kaapa, P.; Zagariya, A. Inflammatory response and apoptosis in newborn lungs after meconium aspiration. Biotechnol. Prog. 2005, 21, 192–197. [Google Scholar] [CrossRef]
- Hofer, N.; Jank, K.; Strenger, V.; Pansy, J.; Resch, B. Inflammatory indices in meconium aspiration syndrome. Pediatr. Pulmonol. 2016, 51, 601–606. [Google Scholar] [CrossRef]
- Dargaville, P.A.; South, M.; McDougall, P.N. Surfactant and surfactant inhibitors in meconium aspiration syndrome. J. Pediatr. 2001, 138, 113–115. [Google Scholar] [CrossRef]
- Davey, A.M.; Becker, J.D.; Davis, J.M. Meconium aspiration syndrome: Physiological and inflammatory changes in a newborn piglet model. Pediatr. Pulmonol. 1993, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- de Beaufort, A.J.; Bakker, A.C.; van Tol, M.J.; Poorthuis, B.J.; Schrama, A.J.; Berger, H.M. Meconium is a source of pro-inflammatory substances and can induce cytokine production in cultured a549 epithelial cells. Pediatr. Res. 2003, 54, 491–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagariya, A.; Bhat, R.; Uhal, B.; Navale, S.; Freidine, M.; Vidyasagar, D. Cell death and lung cell histology in meconium aspirated newborn rabbit lung. Eur. J. Pediatr. 2000, 159, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. The role of nitric oxide synthases in lung inflammation. Curr. Opin. Investig. Drugs 2007, 8, 899–909. [Google Scholar] [PubMed]
- Garthwaite, J.; Boulton, C.L. Nitric oxide signaling in the central nervous system. Annu. Rev. Physiol. 1995, 57, 683–706. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.D.; Carey, R.M.; Kohanski, M.A.; Kennedy, D.W.; Palmer, J.N.; Adappa, N.D.; Cohen, N.A. Relative susceptibility of airway organisms to antimicrobial effects of nitric oxide. Int. Forum Allergy Rhinol. 2017, 7, 770–776. [Google Scholar] [CrossRef]
- Lai, M.Y.; Chu, S.M.; Lakshminrusimha, S.; Lin, H.C. Beyond the inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Pediatr. Neonatol. 2018, 59, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta, E.G.; Diaz, F.J.; Renedo, A.A.; Ruanova, B.F.; de Heredia y Goya, J.L.; Sanchez, L.F.; Valls i Soler, A. Transient response to inhaled nitric oxide in meconium aspiration in newborn lambs. Pediatr. Res. 1998, 43, 198–202. [Google Scholar] [CrossRef] [Green Version]
- Soukka, H.; Viinikka, L.; Kaapa, P. Involvement of thromboxane a2 and prostacyclin in the early pulmonary hypertension after porcine meconium aspiration. Pediatr. Res. 1998, 44, 838–842. [Google Scholar] [CrossRef] [Green Version]
- Holopainen, R.; Aho, H.; Laine, J.; Peuravuori, H.; Soukka, H.; Kaapa, P. Human meconium has high phospholipase a2 activity and induces cellular injury and apoptosis in piglet lungs. Pediatr. Res. 1999, 46, 626–632. [Google Scholar] [CrossRef] [Green Version]
- Mattila, J.T.; Thomas, A.C. Nitric oxide synthase: Non-canonical expression patterns. Front. Immunol. 2014, 5, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nangia, S.; Sunder, S.; Biswas, R.; Saili, A. Endotracheal suction in term non vigorous meconium stained neonates—A pilot study. Resuscitation 2016, 105, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.Y.; Rao, A. Meconium-stained amniotic fluid and meconium aspiration syndrome: A prospective study. Ann. Trop. Paediatr. 2008, 28, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Helsinki Declaration of the World Medical Association (WMA). Ethical principles of medical research involving human subjects. Pol. Merkur Lekarski 2014, 36, 298–301. [Google Scholar]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 852. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, E.; Marin, M.L.; Martin, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernandez, L.; Rodriguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Nagpal, R.; Tsuji, H.; Takahashi, T.; Kawashima, K.; Nagata, S.; Nomoto, K.; Yamashiro, Y. Sensitive quantitative analysis of the meconium bacterial microbiota in healthy term infants born vaginally or by cesarean section. Front. Microbiol. 2016, 7, 1997. [Google Scholar] [CrossRef] [Green Version]
- Stinson, L.F.; Keelan, J.A.; Payne, M.S. Characterization of the bacterial microbiome in first-pass meconium using propidium monoazide (pma) to exclude nonviable bacterial DNA. Lett. Appl. Microbiol. 2019, 68, 378–385. [Google Scholar] [CrossRef]
- Zagariya, A.; Bhat, R.; Chari, G.; Uhal, B.; Navale, S.; Vidyasagar, D. Apoptosis of airway epithelial cells in response to meconium. Life Sci. 2005, 76, 1849–1858. [Google Scholar] [CrossRef]
- WMA Declaration of Taipei on Ethical Considerations Regarding Health Databases and Biobanks. 2016. Available online: https://www.Wma.Net/policies-post/wma-declaration-of-taipei-on-ethical-considerations-regarding-health-databases-and-biobanks/ (accessed on 7 September 2021).
- Galas, R.J., Jr.; Liu, J.C. Surface density of vascular endothelial growth factor modulates endothelial proliferation and differentiation. J. Cell Biochem. 2014, 115, 111–120. [Google Scholar] [CrossRef]
- Patil, R.H.; Naveen Kumar, M.; Kiran Kumar, K.M.; Nagesh, R.; Kavya, K.; Babu, R.L.; Ramesh, G.T.; Chidananda Sharma, S. Dexamethasone inhibits inflammatory response via down regulation of ap-1 transcription factor in human lung epithelial cells. Gene 2018, 645, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Garber, M.; Grabherr, M.G.; Guttman, M.; Trapnell, C. Computational methods for transcriptome annotation and quantification using rna-seq. Nat. Methods 2011, 8, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, D.N.; Garlepp, M.J.; Thompson, P.J. Regulation of the inducible cyclo-oxygenase pathway in human cultured airway epithelial (a549) cells by nitric oxide. Br. J. Pharmacol. 1997, 121, 1482–1488. [Google Scholar] [CrossRef]
- Edwards, E.M.; Lakshminrusimha, S.; Ehret, D.E.Y.; Horbar, J.D. Nicu admissions for meconium aspiration syndrome before and after a national resuscitation program suctioning guideline change. Children 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanaroff, A.A. Meconium aspiration syndrome: Historical aspects. J. Perinatol. 2008, 28 (Suppl. S3), S3–S7. [Google Scholar] [CrossRef] [Green Version]
- Hui, R.; Jing-Jing, P.; Yun-Su, Z.; Xiao-Yu, Z.; Xiao-Qing, C.; Yang, Y. Surfactant lavage for neonatal meconium aspiration syndrome—An updated meta-analysis. J. Chin. Med. Assoc. 2020, 83, 761–773. [Google Scholar] [CrossRef]
- Sun, J.; Qu, S.; Zhang, C.; Xiang, Z.; Fu, Z.; Yao, L. Neonatal mortality rate and risk factors in northeast china: Analysis of 5277 neonates in 2005. Clin. Exp. Obstet. Gynecol. 2014, 41, 512–516. [Google Scholar]
- Whitfield, J.M.; Charsha, D.S.; Chiruvolu, A. Prevention of meconium aspiration syndrome: An update and the baylor experience. Bayl. Univ. Med. Cent. Proc. 2009, 22, 128–131. [Google Scholar] [CrossRef]
- Burke-Strickland, M.; Edwards, N.B. Meconium aspiration in the newborn. Minn. Med. 1973, 56, 1031–1035. [Google Scholar]
- Liu, W.F.; Harrington, T. Delivery room risk factors for meconium aspiration syndrome. Am. J. Perinatol. 2002, 19, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Swarnam, K.; Soraisham, A.S.; Sivanandan, S. Advances in the management of meconium aspiration syndrome. Int. J. Pediatr. 2012, 2012, 359571. [Google Scholar] [CrossRef] [Green Version]
- Gauchan, E.; Basnet, S.; Malla, T. Meconium aspiration syndrome and neonatal outcome: A prospective study. Am. J. Public Health Res. 2015, 3, 48–52. [Google Scholar]
- Louis, D.; Sundaram, V.; Mukhopadhyay, K.; Dutta, S.; Kumar, P. Predictors of mortality in neonates with meconium aspiration syndrome. Indian Pediatr. 2014, 51, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Matalon, R.; Wainstock, T.; Walfisch, A.; Sheiner, E. Exposure to meconium-stained amniotic fluid and long-term neurological-related hospitalizations throughout childhood. Am. J. Perinatol. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Lu, Y.C.; Wang, C.C.; Lee, C.M.; Hwang, K.S.; Hua, Y.M.; Yuh, Y.S.; Chiu, Y.L.; Hsu, W.F.; Chou, Y.L.; Huang, S.W.; et al. Reevaluating reference ranges of oxygen saturation for healthy full-term neonates using pulse oximetry. Pediatr. Neonatol. 2014, 55, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Khazardoost, S.; Hantoushzadeh, S.; Khooshideh, M.; Borna, S. Risk factors for meconium aspiration in meconium stained amniotic fluid. J. Obstet. Gynaecol. 2007, 27, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Kitsommart, R.; Thammawong, N.; Sommai, K.; Yangnoy, J.; Bowornkitiwong, W.; Paes, B. Impact of meconium consistency on infant resuscitation and respiratory outcomes: A retrospective-cohort study and systematic review. J. Matern. Fetal Neonatal Med. 2020, 34, 4141–4147. [Google Scholar] [CrossRef]
- Lama, S.; Mahato, S.K.; Chaudhary, N.; Agrawal, N.; Pathak, S.; Kurmi, O.P.; Bhatia, B.; Agarwal, K.N. Clinico-radiological observations in meconium aspiration syndrome. JNMA J. Nepal Med. Assoc. 2018, 56, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Little, B.B.; Dax, J.S.; Gilstrap, L.C., 3rd; Rosenfeld, C.R. Prediction of the severity of meconium aspiration syndrome. Am. J. Obstet. Gynecol. 1993, 169, 61–70. [Google Scholar] [CrossRef]
- Hovi, M.; Raatikainen, K.; Heiskanen, N.; Heinonen, S. Obstetric outcome in post-term pregnancies: Time for reappraisal in clinical management. Acta Obstet. Gynecol. Scand. 2006, 85, 805–809. [Google Scholar] [CrossRef]
- Maayan-Metzger, A.; Leibovitch, L.; Schushan-Eisen, I.; Strauss, T.; Kuint, J. Meconium-stained amniotic fluid and hypoglycemia among term newborn infants. Fetal Pediatr. Pathol. 2012, 31, 283–287. [Google Scholar] [CrossRef]
- Pariente, G.; Peles, C.; Perri, Z.H.; Baumfeld, Y.; Mastrolia, S.A.; Koifman, A.; Weintraub, A.Y.; Hershkovitz, R. Meconium-stained amniotic fluid—Risk factors and immediate perinatal outcomes among sga infants. J. Matern. Fetal Neonatal Med. 2015, 28, 1064–1067. [Google Scholar] [CrossRef]
- Xu, H.; Mas-Calvet, M.; Wei, S.Q.; Luo, Z.C.; Fraser, W.D. Abnormal fetal heart rate tracing patterns in patients with thick meconium staining of the amniotic fluid: Association with perinatal outcomes. Am. J. Obstet. Gynecol. 2009, 200, 283.e1–283.e7. [Google Scholar] [CrossRef]
- Mazor, M.; Hershkovitz, R.; Bashiri, A.; Maymon, E.; Schreiber, R.; Dukler, D.; Katz, M.; Shoham-Vardi, I. Meconium stained amniotic fluid in preterm delivery is an independent risk factor for perinatal complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 81, 9–13. [Google Scholar] [CrossRef]
- Wiswell, T.E.; Bent, R.C. Meconium staining and the meconium aspiration syndrome. Unresolved issues. Pediatr. Clin. N. Am. 1993, 40, 955–981. [Google Scholar] [CrossRef]
- Katz, L.A.; Klein, J.M. Repeat surfactant therapy for postsurfactant slump. J. Perinatol. 2006, 26, 414–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, M.J.; Soong, W.J.; Lee, Y.S.; Tsao, P.C.; Yang, C.F.; Chiu, S.Y.; Tang, R.B. Meconium exposure dependent cell death and apoptosis in human alveolar epithelial cells. Pediatr. Pulmonol. 2010, 45, 816–823. [Google Scholar] [CrossRef]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the a549 cell line as a type ii pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef]
- Korhonen, K.; Soukka, H.; Halkola, L.; Peuravuori, H.; Aho, H.; Pulkki, K.; Kero, P.; Kaapa, P.O. Meconium induces only localized inflammatory lung injury in piglets. Pediatr. Res. 2003, 54, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Porta, N.F.; Steinhorn, R.H. Pulmonary vasodilator therapy in the nicu: Inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin. Perinatol. 2012, 39, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Nathan, C.; Xie, Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Eberhardt, W.; Beck, K.F. Regulation of gene expression by nitric oxide. Pflug. Arch. 2001, 442, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Fontanilla, R.; Zagariya, A.; Vidyasagar, D. Meconium-induced release of nitric oxide in rabbit alveolar cells. J. Perinatol. 2008, 28 (Suppl. S3), S123–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.M.; Lally, K.P.; Elidemir, O.; Colasurdo, G.N. Meconium enhances the release of nitric oxide in human airway epithelial cells. Biol. Neonate 2002, 81, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kytola, J.; Uotila, P.; Kaapa, P. Meconium stimulates cyclooxygenase-2 expression in rat lungs. Prostaglandins Leukot. Essent. Fat. Acids 1999, 60, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Yan, Z.Q.; Brauner, A.; Tullus, K. Meconium induces expression of inducible no synthase and activation of nf-kappab in rat alveolar macrophages. Pediatr. Res. 2001, 49, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, N.K.; Burns, K.E.; Friedrich, J.O.; Granton, J.T.; Cook, D.J.; Meade, M.O. Effect of nitric oxide on oxygenation and mortality in acute lung injury: Systematic review and meta-analysis. BMJ 2007, 334, 779. [Google Scholar] [CrossRef] [Green Version]
- Crosswhite, P.; Sun, Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J. Hypertens. 2010, 28, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A. Nitric oxide synthase and cyclooxygenase pathways: A complex interplay in cellular signaling. Curr. Med. Chem. 2016, 23, 2559–2578. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Salvemini, D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007, 71, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uotila, P.J.; Kaapa, P.O. Cyclooxygenase-2 expression in human monocytes stimulated by meconium. Lancet 1998, 351, 878. [Google Scholar] [CrossRef]
- Fan, H.C.; Wang, S.Y.; Peng, Y.J.; Lee, H.S. Valproic acid impacts the growth of growth plate chondrocytes. Int. J. Environ. Res. Public Health 2020, 17, 3675. [Google Scholar] [CrossRef]
- Robbins, R.A.; Springall, D.R.; Warren, J.B.; Kwon, O.J.; Buttery, L.D.; Wilson, A.J.; Adcock, I.M.; Riveros-Moreno, V.; Moncada, S.; Polak, J.; et al. Inducible nitric oxide synthase is increased in murine lung epithelial cells by cytokine stimulation. Biochem. Biophys. Res. Commun. 1994, 198, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Belvisi, M.; Barnes, P.J.; Larkin, S.; Yacoub, M.; Tadjkarimi, S.; Williams, T.J.; Mitchell, J.A. Nitric oxide synthase activity is elevated in inflammatory lung disease in humans. Eur. J. Pharmacol. 1995, 283, 255–258. [Google Scholar] [CrossRef]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles prize lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Ward, M.; Sinn, J. Steroid therapy for meconium aspiration syndrome in newborn infants. Cochrane Database Syst. Rev. 2003, 2003, CD003485. [Google Scholar] [CrossRef]
- Yeung, T.; Jasani, B.; Shah, P.S. Steroids for the management of neonates with meconium aspiration syndrome: A systematic review and meta-analysis. Indian Pediatr. 2021, 58, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumar, A.; Bhatia, B.D.; Satya, K.; Singh, T.B. Role of steroids on the clinical course and outcome of meconium aspiration syndrome—A randomized controlled trial. J. Trop. Pediatr. 2007, 53, 331–337. [Google Scholar] [CrossRef]
- Tripathi, S.; Saili, A. The effect of steroids on the clinical course and outcome of neonates with meconium aspiration syndrome. J. Trop. Pediatr. 2007, 53, 8–12. [Google Scholar] [CrossRef]
- Tripathi, S.; Saili, A.; Dutta, R. Inflammatory markers in meconium induced lung injury in neonates and effect of steroids on their levels: A randomized controlled trial. Indian J. Med. Microbiol. 2007, 25, 103–107. [Google Scholar] [CrossRef]
- Mikolka, P.; Mokra, D.; Kopincova, J.; Tomcikova-Mikusiakova, L.; Calkovska, A. Budesonide added to modified porcine surfactant curosurf may additionally improve the lung functions in meconium aspiration syndrome. Physiol. Res. 2013, 62, S191–S200. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Mokry, J.; Drgova, A.; Petraskova, M.; Bulikova, J.; Calkovska, A. Intratracheally administered corticosteroids improve lung function in meconium-instilled rabbits. J. Physiol. Pharmacol. 2007, 58 (Suppl. S5), 389–398. [Google Scholar]
- Hong, H.; Jang, B.C. Prednisone inhibits the il-1beta-induced expression of cox-2 in hei-oc1 murine auditory cells through the inhibition of erk-1/2, jnk-1 and ap-1 activity. Int. J. Mol. Med. 2014, 34, 1640–1646. [Google Scholar] [CrossRef]
- Linehan, J.D.; Kolios, G.; Valatas, V.; Robertson, D.A.; Westwick, J. Effect of corticosteroids on nitric oxide production in inflammatory bowel disease: Are leukocytes the site of action? Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G261–G267. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the role of nitric oxide and its clinical applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Bryan, N.S. Nitrite in nitric oxide biology: Cause or consequence? A systems-based review. Free Radic. Biol. Med. 2006, 41, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Pechkovsky, D.V.; Zissel, G.; Goldmann, T.; Einhaus, M.; Taube, C.; Magnussen, H.; Schlaak, M.; Muller-Quernheim, J. Pattern of nos2 and nos3 mrna expression in human a549 cells and primary cultured aec ii. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L684–L692. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene Name | Sequence | Product (bp) | RefSeq No. |
|---|---|---|---|
| COX-2 | Probe 56FAM 5′-ACATCCAGA-ZEN-TCACATTTGATTGACAGTCCA-3IABkFQ-3’ | 30 | NM_000963 |
| 5′- GCCATAGTCAGCATTGTAAGTTG -3′ | |||
| 5′- GCACTACATACTTACCCACTTCA -3′ | |||
| NOS-1 | Probe 56FAM 5′-TCCTTAGCC-ZEN-GTCAAAACCTCCAGAG-3IABkFQ-32032 | 25 | NM_000963 |
| 5′- AGACGCACGAAGATAGTTGAC-3′ | |||
| 5′- CCGAAGCTCCAGAACTCAC-3′ | |||
| NOS-2 | Probe 56FAM 5′- TATTCAGCT -ZEN- GTGCCTTCAACCCCA -3IABkFQ-3′ | 24 | NM_000625 |
| 5′- GCAGCTCAGCCTGTACT-3′ | |||
| 5′- CACCATCCTCTTTGCGACA-3′ | |||
| NOS-3 | Probe 56FAM 5′- TATTCAGCT -ZEN- GTGCCTTCAACCCCA -3IABk FQ-3′ | 23 | NM_001160110 |
| 5′-ACGATGGTGACTTTGGCTA-3′ | |||
| 5′-TGGAGGATGTGGCTGTCT-3′ | |||
| B2M | Probe 56FAM 5′- CCTGCCGTG -ZEN- TGAACCATGTGACT -3IABkFQ -3′ | 23 | 99832111 |
| 5′- ACCTCCATGATGCTGCTTAC -3′ | |||
| 5′- GGACTGGTCTTTCTATCTCTTGT -3′ |
| Thin Meconium N = 72 | Thick Meconium N = 23 | p-Value | |
|---|---|---|---|
| Maternal factors | |||
| Maternal age, years | 30.38 ± 4.39 | 31.13 ± 5.41 | 0.46 |
| Delivery mode, (CS/NSD) | 17/55 | 8/15 | 0.29 |
| Preeclampsia, N (%) | 5 (6.94%) | 2 (8.70%) | 0.68 |
| Diabetes, N (%) | 6 (8.33%) | 2 (8.70%) | 1.00 |
| Antepartum hemorrhage, N (%) | 2 (2.78%) | 1 (4.35%) | 0.57 |
| PROM, N (%) | 8 (11.11%) | 3 (13.04%) | 0.72 |
| Polyhydramnios, N (%) | 4 (5.56%) | 2 (8.70%) | 0.35 |
| Oligohydramnios, N (%) | 3 (4.17%) | 1 (4.35%) | 1.00 |
| Neonatal factors | |||
| Gestational age, weeks | 39.39 ± 3.01 | 39.13 ± 2.82 | 0.51 |
| Birth weight, g | 3039.24 ± 497.19 | 2836.35 ± 490.40 | 0.09 |
| Sex (female/male) | 36/36 | 12/11 | 1.00 |
| APGAR1 min | 7.80 ± 1.31 | 6.19 ± 2.64 | 0.01 * |
| APGAR5 min | 9.01 ± 0.83 | 7.86 ± 2.22 | 0.02 * |
| Hypoglycemia, N (%) | 3 (4.17%) | 2 (8.70%) | 1.00 |
| NICU admission, N (%) | 0 | 12 (52.17%) | <0.001 ** |
| CPAP, N (%) | 0 | 6 (26.09%) | <0.001 ** |
| Intubation, N (%) | 0 | 7 (30.43%) | <0.001 ** |
| Ventilator, N (%) | 0 | 6 (26.09%) | <0.001 ** |
| Death, N (%) | 0 | 2 (8.70%) | 0.06 |
| Cell Lines | A549 | HEL299 | ||
|---|---|---|---|---|
| Name/Gene ID/MIM | Gene Description | Fold Increase | Map | |
| NOS1/4842/163731 | nitric oxide synthase 1(NOS-1) | 0.9845475 | 1.6171549 | 12q24.22 |
| NOS2/4843/163730 | nitric oxide synthase 2 (NOS-2) | 0.4949685 | 3.2921734 | 17q11.2 |
| NOS3/4846/163729 | nitric oxide synthase 3 (NOS-3) | 1.1372183 | 1.1059593 | 7q36.1 |
| PTGS2/5743/600262 | cyclooxygenase-2 (COX-2) | 22.952443 | 19.439566 | 1q31.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.-C.; Chang, F.-W.; Pan, Y.-R.; Yu, S.-I.; Chang, K.-H.; Chen, C.-M.; Liu, C.-A. Approach to the Connection between Meconium Consistency and Adverse Neonatal Outcomes: A Retrospective Clinical Review and Prospective In Vitro Study. Children 2021, 8, 1082. https://doi.org/10.3390/children8121082
Fan H-C, Chang F-W, Pan Y-R, Yu S-I, Chang K-H, Chen C-M, Liu C-A. Approach to the Connection between Meconium Consistency and Adverse Neonatal Outcomes: A Retrospective Clinical Review and Prospective In Vitro Study. Children. 2021; 8(12):1082. https://doi.org/10.3390/children8121082
Chicago/Turabian StyleFan, Hueng-Chuen, Fung-Wei Chang, Ying-Ru Pan, Szu-I Yu, Kuang-Hsi Chang, Chuan-Mu Chen, and Ching-Ann Liu. 2021. "Approach to the Connection between Meconium Consistency and Adverse Neonatal Outcomes: A Retrospective Clinical Review and Prospective In Vitro Study" Children 8, no. 12: 1082. https://doi.org/10.3390/children8121082