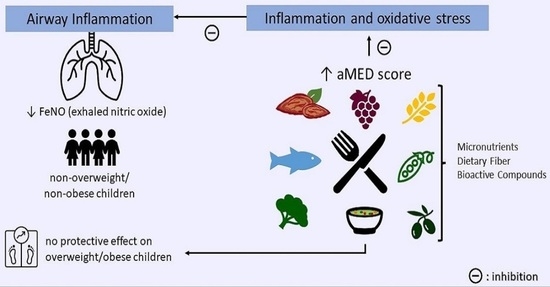
Mediterranean Diet and Airway Inflammation in School-Aged Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Participants Assessment
2.2.1. Dietary and Diet Quality Assessment
2.2.2. Anthropometry
2.2.3. Airway Inflammation
2.2.4. Covariates
2.2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Tenero, L.; Zaffanello, M.; Piazza, M.; Piacentini, G. Measuring Airway Inflammation in Asthmatic Children. Front. Pediatr. 2018, 6, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Kudo, M.; Ishigatsubo, Y.; Aoki, I. Pathology of asthma. Front. Microbiol. 2013, 4, 263. [Google Scholar] [CrossRef] [Green Version]
- Pijnenburg, M.W.; De Jongste, J.C. Exhaled nitric oxide in childhood asthma: A review. Clin. Exp. Allergy 2008, 38, 246–259. [Google Scholar] [CrossRef]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Sharma, V.; Cowan, D.C. Obesity, Inflammation, and Severe Asthma: An Update. Curr. Allergy Asthma Rep. 2021, 21, 46. [Google Scholar] [CrossRef]
- Sansone, F.; Attanasi, M.; Di Pillo, S.; Chiarelli, F. Asthma and Obesity in Children. Biomedicines 2020, 8, 231. [Google Scholar] [CrossRef]
- Vezir, E.; Civelek, E.; Dibek Misirlioglu, E.; Toyran, M.; Capanoglu, M.; Karakus, E.; Kahraman, T.; Ozguner, M.; Demirel, F.; Gursel, I.; et al. Effects of Obesity on Airway and Systemic Inflammation in Asthmatic Children. Int. Arch. Allergy Immunol. 2021, 182, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Shore, S.A. Adiponectin, Leptin, and Resistin in Asthma: Basic Mechanisms through Population Studies. J. Allergy 2013, 2013, 785835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Halnes, I.; Baines, K.J.; Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients 2017, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef] [Green Version]
- Shore, S.A.; Cho, Y. Obesity and Asthma: Microbiome-Metabolome Interactions. Am. J. Respir. Cell Mol. Biol. 2016, 54, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.G.; Gibson, P.G. Dietary factors lead to innate immune activation in asthma. Pharmacol. Ther. 2009, 123, 37–53. [Google Scholar] [CrossRef]
- Andrianasolo, R.M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Galan, P.; Varraso, R. Associations between dietary scores with asthma symptoms and asthma control in adults. Eur. Respir. J. 2018, 52, 1702572. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Angel, J.; Han, Y.Y.; Litonjua, A.A.; Celedón, J.C. Diet and asthma: Is the sum more important than the parts? J. Allergy Clin. Immunol. 2021, 148, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.K.; Thien, F.C.; Abramson, M.J. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst. Rev. 2002, CD001283. [Google Scholar] [CrossRef]
- Ardern, K.D. Dietary salt reduction or exclusion for allergic asthma. Cochrane Database Syst. Rev. 2004, CD000436. [Google Scholar] [CrossRef]
- Anandan, C.; Nurmatov, U.; Sheikh, A. Omega 3 and 6 oils for primary prevention of allergic disease: Systematic review and meta-analysis. Allergy 2009, 64, 840–848. [Google Scholar] [CrossRef]
- Pogson, Z.; McKeever, T. Dietary sodium manipulation and asthma. Cochrane Database Syst. Rev. 2011, 2011, CD000436. [Google Scholar] [CrossRef]
- Yang, H.; Xun, P.; He, K. Fish and fish oil intake in relation to risk of asthma: A systematic review and meta-analysis. PLoS ONE 2013, 8, e80048. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Morrissey, E.; Giltinan, M.; Kehoe, L.; Nugent, A.P.; McNulty, B.A.; Flynn, A.; Walton, J. Sodium and Potassium Intakes and Their Ratio in Adults (18-90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 2020, 12, 938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Koumpagioti, D.; Boutopoulou, B.; Moriki, D.; Priftis, K.N.; Douros, K. Does Adherence to the Mediterranean Diet Have a Protective Effect against Asthma and Allergies in Children? A Systematic Review. Nutrients 2022, 14, 1618. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.C.; Paciência, I.; Cavaleiro Rufo, J.; Silva, D.; Delgado, L.; Moreira, A.; Moreira, P. Dietary Acid Load Modulation of Asthma-Related miRNAs in the Exhaled Breath Condensate of Children. Nutrients 2022, 14, 1147. [Google Scholar] [CrossRef]
- Mendes, F.C.; Paciência, I.; Cavaleiro Rufo, J.; Farraia, M.; Silva, D.; Padrão, P.; Delgado, L.; Garcia-Larsen, V.; Moreira, A.; Moreira, P. Higher diversity of vegetable consumption is associated with less airway inflammation and prevalence of asthma in school-aged children. Pediatr. Allergy Immunol. 2021, 32, 925–936. [Google Scholar] [CrossRef]
- Rodrigues, M.; de Castro Mendes, F.; Paciência, I.; Cavaleiro Rufo, J.; Silva, D.; Delgado, L.; Moreira, A.; Moreira, P. Diet Quality and Exhaled Breath Condensate Markers in a Sample of School-Aged Children. Children 2023, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; de Castro Mendes, F.; Paciência, I.; Barros, R.; Padrão, P.; Cavaleiro Rufo, J.; Silva, D.; Delgado, L.; Moreira, A.; Moreira, P. Diet quality, asthma and airway inflammation in school-aged children. Eur. Ann. Allergy Clin. Immunol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- El Kinany, K.; Mint Sidi Deoula, M.; Hatime, Z.; Boudouaya, H.A.; Atassi, M.; El Asri, A.; Benslimane, A.; Nejjari, C.; Ibrahimi, S.A.; Lagiou, P.; et al. Modified Mediterranean diet score adapted to a southern Mediterranean population and its relation to overweight and obesity risk. Public Health Nutr. 2021, 24, 4064–4070. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Fonseca, J.; de Oliveira, J.F.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy 2008, 63, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Lagiou, P. Healthy traditional Mediterranean diet: An expression of culture, history, and lifestyle. Nutr. Rev. 1997, 55 Pt 1, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef]
- Douros, K.; Thanopoulou, M.I.; Boutopoulou, B.; Papadopoulou, A.; Papadimitriou, A.; Fretzayas, A.; Priftis, K.N. Adherence to the Mediterranean diet and inflammatory markers in children with asthma. Allergol. Immunopathol. 2019, 47, 209–213. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Katsardis, C.; Lambert, K.; Tsoukalas, D.; Koutsilieris, M.; Erbas, B.; Itsiopoulos, C. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: A randomised controlled trial. J. Hum. Nutr. Diet. 2019, 32, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.L.; Ardouin, S.; Burrows, T. The validity of dietary assessment methods to accurately measure energy intake in children and adolescents who are overweight or obese: A systematic review. Eur. J. Clin. Nutr. 2018, 72, 185–197. [Google Scholar] [CrossRef]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC Growth Charts: United States; Department of Health and Human Services: Washington, DC, USA, 2000; pp. 1–27.
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.C.; Plummer, A.L.; Taylor, D.R. An official ATS clinical practice guideline: Interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Nurmatov, U.; Nwaru, B.I.; Devereux, G.; Sheikh, A. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy 2012, 67, 1041–1059. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Greenhawt, M.; Meyer, R.W.; Agostoni, C.; Reese, I.; du Toit, G.; Feeney, M.; Maslin, K.; Nwaru, B.I.; Roduit, C.; et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: Novel concepts and implications for studies in allergy and asthma. Allergy 2020, 75, 497–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharitonov, S.A.; Robbins, R.A.; Yates, D.; Keatings, V.; Barnes, P.J. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am. J. Respir. Crit. Care Med. 1995, 152, 609–612. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Elsenburg, L.K.; Rieckmann, A.; Nguyen, T.-L.; Bengtsson, J.; Andersen, A.-M.N.; Taylor-Robinson, D.; Lange, T.; Rod, N.H. Mediation of the parental education gradient in early adult mortality by childhood adversity: A population-based cohort study of more than 1 million children. Lancet Public Health 2022, 7, e146–e155. [Google Scholar] [CrossRef]
- Amazouz, H.; Roda, C.; Beydon, N.; Lezmi, G.; Bourgoin-Heck, M.; Just, J.; Momas, I.; Rancière, F. Mediterranean diet and lung function, sensitization, and asthma at school age: The PARIS cohort. Pediatr. Allergy Immunol. 2021, 32, 1437–1444. [Google Scholar] [CrossRef]
- Cardinale, F.; Tesse, R.; Fucilli, C.; Loffredo, M.S.; Iacoviello, G.; Chinellato, I.; Armenio, L. Correlation between exhaled nitric oxide and dietary consumption of fats and antioxidants in children with asthma. J. Allergy Clin. Immunol. 2007, 119, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Tastekin, E.; Palabiyik, O.; Ulucam, E.; Uzgur, S.; Karaca, A.; Vardar, S.A.; Yilmaz, A.; Aydogdu, N. The effect of high protein diet and exercise on irisin, eNOS, and iNOS expressions in kidney. Ren. Fail. 2016, 38, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Fitó, M.; Morales-Suárez-Varela, M.; Moya, A.; Gómez, S.F.; Schröder, H. Mediterranean diet and adiposity in children and adolescents: A systematic review. Obes. Rev. 2022, 23 (Suppl. S1), e13381. [Google Scholar] [CrossRef]
- Rodrigues, M.; de Castro Mendes, F.; Delgado, L.; Padrão, P.; Paciência, I.; Barros, R.; Rufo, J.C.; Silva, D.; Moreira, A.; Moreira, P. Diet and Asthma: A Narrative Review. Appl. Sci. 2023, 13, 6398. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Holguin, F. A review of obesity and asthma across the life span. J. Asthma 2018, 55, 1286–1300. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, A.; Fonseca, J.; Moreira, P.; Fernandes, L.; de Oliveira, J.F.; Delgado, L.; Castel-Branco, M.G. Obesity and airway inflammation in asthma. J. Allergy Clin. Immunol. 2006, 117, 1501–1502. [Google Scholar] [CrossRef]
- Flashner, B.M.; Rifas-Shiman, S.L.; Oken, E.; Camargo, C.A.; Platts-Mills, T.J.; Workman, L.; Litonjua, A.A.; Gold, D.R.; Rice, M.B. Obesity, sedentary lifestyle, and exhaled nitric oxide in an early adolescent cohort. Pediatr. Pulmonol. 2020, 55, 503–509. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Katsardis, C.; Tsoukalas, D.; Erbas, B.; Itsiopoulos, C. Weight Status and Respiratory Health in Asthmatic Children. Lung 2019, 197, 777–782. [Google Scholar] [CrossRef]
- Maniscalco, M.; de Laurentiis, G.; Zedda, A.; Faraone, S.; Giardiello, C.; Cristiano, S.; Sofia, M. Exhaled nitric oxide in severe obesity: Effect of weight loss. Respir. Physiol. Neurobiol. 2007, 156, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Weiner, D.J.; Mullen, J.; Sawicki, G.; Kurland, G.; Han, Y.Y.; Cloutier, M.M.; Canino, G.; Weiss, S.T.; Litonjua, A.A.; et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am. J. Respir. Crit. Care Med. 2017, 195, 314–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holguin, F. Arginine and nitric oxide pathways in obesity-associated asthma. J. Allergy 2013, 2013, 714595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, R.; Moreira, A.; Fonseca, J.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Dietary intake of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011, 106, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef]
- Savitz, D.A.; Wellenius, G.A. Can Cross-Sectional Studies Contribute to Causal Inference? It Depends. Am. J. Epidemiol. 2022, 192, 514–516. [Google Scholar] [CrossRef]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [Green Version]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31 (Suppl. S3), 38–45. [Google Scholar]
- Foster, E.; Bradley, J. Methodological considerations and future insights for 24-hour dietary recall assessment in children. Nutr. Res. 2018, 51, 1–11. [Google Scholar] [CrossRef]
- Biró, G.; Hulshof, K.F.; Ovesen, L.; Amorim Cruz, J.A. Selection of methodology to assess food intake. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S2), S25–S32. [Google Scholar] [CrossRef] [Green Version]
- Wolper, C.; Heshka, S.; Heymsfield, S.B. Measuring Food Intake: An Overview. In Handbook of Assessment Methods for Eating Behaviors and Weight-Related Problems: Measures, Theory, and Research; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1995. [Google Scholar]
- Müller, M.J.; Lagerpusch, M.; Enderle, J.; Schautz, B.; Heller, M.; Bosy-Westphal, A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes. Rev. 2012, 13 (Suppl. S2), 6–13. [Google Scholar] [CrossRef] [PubMed]
- Curry, B.A.; Blizzard, C.L.; Schmidt, M.D.; Walters, E.H.; Dwyer, T.; Venn, A.J. Longitudinal associations of adiposity with adult lung function in the Childhood Determinants of Adult Health (CDAH) study. Obesity 2011, 19, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Papoutsakis, C.; Priftis, K.N.; Drakouli, M.; Prifti, S.; Konstantaki, E.; Chondronikola, M.; Antonogeorgos, G.; Matziou, V. Childhood Overweight/Obesity and Asthma: Is There a Link? A Systematic Review of Recent Epidemiologic Evidence. J. Acad. Nutr. Diet. 2013, 113, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Delgado, L. Visceral adipose tissue: A clue to the obesity-asthma endotype(s)? Rev. Port. Pneumol. 2016, 22, 253–254. [Google Scholar] [CrossRef]
- Lundahl, A.; Kidwell, K.M.; Nelson, T.D. Parental underestimates of child weight: A meta-analysis. Pediatrics 2014, 133, e689–e703. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.; Severo, M.; Paciência, I.; Rufo, J.; Martins, C.; Moreira, P.; Padrão, P.; Delgado, L.; Moreira, A. Setting definitions of childhood asthma in epidemiologic studies. Pediatr. Allergy Immunol. 2019, 30, 708–715. [Google Scholar] [CrossRef]
- Rodrigues, S.S.P.; Franchini, B.M.; Pinho, I.S.M.; Graça, A. The Portuguese mediterranean diet wheel: Development considerations. Br. J. Nutr. 2022, 128, 1315–1321. [Google Scholar] [CrossRef]

| Total, n = 660 (100%) | Fractional eNO < 35 ppb, n = 574 (87%) | Fractional eNO ≥ 35 ppb, n = 86 (13%) | p-Value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 8.68 ± 0.77 | 8.66 ± 0.77 | 8.85 ± 0.75 | 0.041 |
| Sex, female, n (%) | 324 (49.1%) | 288 (50.2%) | 36 (41.9%) | 0.15 |
| BMI | 0.034 | |||
| Non-overweight/non-obese (p < 85th) | 491 (74.4%) | 419 (73%) | 72 (87.7%) | |
| Overweight/obese (p ≥ 85th) | 169 (25.6%) | 155 (27%) | 14 (16.3%) | |
| aMED score, mean ± SD | 2.76 ± 1.48 | 2.80 ± 1.46 | 2.52 ± 1.57 | 0.11 |
| Vegetables (g), median (25th–75th) | 87.12 (34.27–150.77) | 87.12 (29.54–149.14) | 87.12 (45.04–165.70) | 0.92 |
| Fruits (g), median (25th–75th) | 174.0 (84.0–303.5) | 174.0 (84.0–302.0) | 174.0 (74.00–305.75) | 0.48 |
| Ratio MUFA/SFA | 1.17 (0.92–1.45) | 1.18 (0.92–1.46) | 1.11 (0.85–1.33) | 0.050 |
| Fish (g), median (25th–75th) | 0.00 (0.00–100.00) | 0.00 (0.00–100.00) | 0.00 (0.00–62.50) | 0.19 |
| Nuts (g), median (25th–75th) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.37 |
| Whole grains (g), median (25th–75th) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.35 |
| Legumes (g), median (25th–75th) | 0.00 (0.00–0.00) | 0.00 (0.00–9.76) | 0.00 (0.00–0.00) | 0.13 |
| Red and processed meat (g), median (25th–75th) | 74.8 (20.0–140.0) | 72.65 (20.0–140.0) | 84.85 (20.0–138.83) | 0.65 |
| Total energy intake (kcal), median (25th–75th) | 2164.75 (1867.95; 2476.06) | 2176.93 (1872.69–2492.47) | 2081.45 (1778.45–2436.30) | 0.19 |
| Breastfeeding a, n (%) | 474 (71.8%) | 350 (71.3%) | 124 (73.4%) | 0.28 |
| Tobacco exposure b, n (%) | 144 (24.0%) | 127 (24.2%) | 17 (22.4%) | 0.73 |
| Atopy c, n (%) | 227 (34.9%) | 172 (30.4%) | 55 (64.7%) | 0.001 |
| Parental education d, n (%) | 0.73 | |||
| <9 years | 188 (28.5%) | 161 (28%) | 27 (31.4%) | |
| 10–12 years | 161 (24.4%) | 141 (24.6%) | 20 (23.3%) | |
| >12 years | 180 (27.3%) | 159 (27.7%) | 21 (24.4%) | |
| Total, n = 660 (100%) | Non-Overweight/Non-Obese, n = 491 (74.4%) | Overweight/Obese, n = 169 (25.6%) | p-Value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 8.68 ± 0.77 | 8.66 ± 0.76 | 8.76 ± 0.81 | 0.15 |
| Sex, female, n (%) | 324 (49.1%) | 242 (49.3%) | 82 (48.5%) | 0.86 |
| aMED score, mean ± SD | 2.76 ± 1.48 | 2.79 ± 1.51 | 2.69 ± 1.39 | 0.44 |
| Vegetables (g), median (25th–75th) | 87.12 (34.27–150.77) | 87.12 (44.04–153.60) | 87.12 (29.07–149.21) | 0.37 |
| Fruits (g), median (25th–75th) | 174.0 (84.0–303.5) | 174.0 (84.00–308.00) | 174.0 (80.0–300.0) | 0.55 |
| Ratio MUFA/SFA | 1.17 (0.92–1.45) | 1.18 (0.92–1.47) | 1.14 (0.92–1.43) | 0.89 |
| Fish (g), median (25th–75th) | 0.00 (0.00–100.00) | 0.00 (0.00–100.00) | 0.00 (0.00–100.00) | 0.77 |
| Nuts (g), median (25th–75th) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.43 |
| Whole grains (g), median (25th–75th) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.69 |
| Legumes (g), median (25th–75th) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–9.84) | 0.74 |
| Red and processed meat (g), median (25th–75th) | 74.8 (20.0–140.0) | 73.20 (20.00–141.00) | 76.00 (20.00–140.00) | 0.58 |
| Total energy intake (kcal), median (25th–75th) | 2164.75 (1867.95; 2476.06) | 2135.95 (1848.18–2468.38) | 2241.10 (1928.69–2519.82) | 0.049 |
| Breastfeeding a, n (%) | 474 (71.8%) | 350 (81.4%) | 124 (83.2%) | 0.62 |
| Tobacco exposure b, n (%) | 144 (24.0%) | 102 (22.8%) | 42 (27.5%) | 0.24 |
| Atopy c, n (%) | 227 (34.9%) | 173 (35.7%) | 54 (32.3%) | 0.43 |
| Parental education d, n (%) | 0.021 | |||
| <9 years | 188 (28.5%) | 131 (33.2%) | 57 (42.2%) | |
| 10–12 years | 161 (24.4%) | 116 (29.4%) | 45 (33.3%) | |
| >12 years | 180 (27.3%) | 147 (37.3%) | 33 (24.4%) |
| aMED Score Crude Model, OR (95% CI) | p-Value | aMED Score Adjusted Model, OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Increased Levels of Exhaled Nitric Oxide (≥35 ppb) | ||||
| All participants | 0.88 (0.75–1.03) | 0.109 | 0.84 (0.69–1.02) | 0.08 |
| Non-overweight/non-obese | 0.85 (0.72–1.00) | 0.055 | 0.77 (0.61–0.97) | 0.025 |
| Overweight/obese | 1.06 (0.71–1.57) | 0.779 | 1.57 (0.88–2.79) | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.; de Castro Mendes, F.; Padrão, P.; Delgado, L.; Paciência, I.; Barros, R.; Rufo, J.C.; Silva, D.; Moreira, A.; Moreira, P. Mediterranean Diet and Airway Inflammation in School-Aged Children. Children 2023, 10, 1305. https://doi.org/10.3390/children10081305
Rodrigues M, de Castro Mendes F, Padrão P, Delgado L, Paciência I, Barros R, Rufo JC, Silva D, Moreira A, Moreira P. Mediterranean Diet and Airway Inflammation in School-Aged Children. Children. 2023; 10(8):1305. https://doi.org/10.3390/children10081305
Chicago/Turabian StyleRodrigues, Mónica, Francisca de Castro Mendes, Patrícia Padrão, Luís Delgado, Inês Paciência, Renata Barros, João Cavaleiro Rufo, Diana Silva, André Moreira, and Pedro Moreira. 2023. "Mediterranean Diet and Airway Inflammation in School-Aged Children" Children 10, no. 8: 1305. https://doi.org/10.3390/children10081305