A Recombinant Peptide Device Combined with Adipose Tissue-Derived Stem Cells Enhances Subcutaneous Islet Engraftment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. The Isolation and Culture of ADSCs
2.3. Flow Cytometry
2.4. In Vivo Bioluminescence Imaging of ADSCs Seeded on RCP Devices
2.5. Preparation and Implantation of RCP Devices with/without ADSCs
2.6. Islet Isolation and Transplantation
2.7. The Induction and Diagnosis of Diabetes in the Recipients
2.8. Intraperitoneal Glucose Tolerance Test
2.9. Immunohistochemical Staining
2.10. Statistical Analyses
3. Results
3.1. Characterization of ADSCs
3.2. The Luciferase Expression of Transplanted Bioluminescent ADSCs
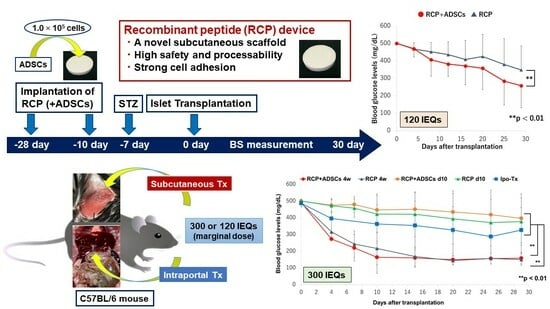
3.3. The Outcome of Islet Engraftment after Syngeneic Mouse Islet Transplantation
3.4. Intraperitoneal Glucose Tolerance Test
3.5. State of Neovascularization and Apoptosis around Transplanted Islet Grafts
3.6. Immunohistochemical staining of Extracellular Matrices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Goto, M.; Johansson, H.; Maeda, A.; Elgue, G.; Korsgren, O.; Nilsson, B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation 2004, 77, 741–747. [Google Scholar] [CrossRef]
- Yin, D.; Ding, J.W.; Shen, J.; Ma, L.; Hara, M.; Chong, A.S. Liver ischemia contributes to early islet failure following intraportal transplantation: Benefits of liver ischemic-preconditioning. Am J Transpl. 2006, 6, 60–68. [Google Scholar] [CrossRef]
- Ryan, E.A.; Lakey, J.R.; Paty, B.W.; Imes, S.; Korbutt, G.S.; Kneteman, N.M.; Bigam, D.; Rajotte, R.V.; Shapiro, A.M. Successful islet transplantation: Continued insulin reserve provides long-term glycemic control. Diabetes 2002, 51, 2148–2157. [Google Scholar] [CrossRef]
- Rajab, A. Islet transplantation: Alternative sites. Curr. Diabetes Rep. 2010, 10, 332–337. [Google Scholar] [CrossRef]
- Sakata, N.; Aoki, T.; Yoshimatsu, G.; Tsuchiya, H.; Hata, T.; Katayose, Y.; Egawa, S.; Unno, M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab. Res. Rev. 2014, 30, 1–10. [Google Scholar] [CrossRef]
- Kemp, C.B.; Knight, M.J.; Scharp, D.W.; Ballinger, W.F.; Lacy, P.E. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia 1973, 9, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Bonner-Weir, S.; Ogawa, Y.; Vacanti, J.P.; Weir, G.C. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation 1996, 61, 1557–1561. [Google Scholar] [CrossRef]
- Schaffler, A.; Buchler, C. Concise review: Adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.H.; Kim, S.Y.; Kim, Y.J.; Kim, S.J.; Lee, J.B.; Bae, Y.C.; Sung, S.M.; Jung, J.S. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2006, 17, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Zhao, R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.S.; Inagaki, A.; Nakamura, Y.; Imura, T.; Igarashi, Y.; Fathi, I.; Miyagi, S.; Ohuchi, N.; Satomi, S.; Goto, M. The Optimization of the Prevascularization Procedures for Improving Subcutaneous Islet Engraftment. Transplantation 2018, 102, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Bayne, K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. Physiologist 1996, 39, 199, 208–211. [Google Scholar] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, Y.; Tanemura, M.; Kawaguchi, N.; Machida, T.; Tanida, T.; Deguchi, T.; Wada, H.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; et al. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation 2010, 90, 1366–1373. [Google Scholar] [CrossRef]
- Nakamura, K.; Tabata, Y. A new fluorescent imaging of renal inflammation with RCP. J. Control Release 2010, 148, 351–358. [Google Scholar] [CrossRef]
- Nakamura, K.; Iwazawa, R.; Yoshioka, Y. Introduction to a new cell transplantation platform via recombinant peptide petaloid pieces and its application to islet transplantation with mesenchymal stem cells. Transpl. Int. 2016, 29, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Nishioka, S.; Fujisawa, I.; Shiku, H.; Shimada, M.; Sekiguchi, S.; Fujimori, K.; Ushiyama, A.; Matsue, T.; Ohuchi, N.; et al. Tacrolimus inhibits the revascularization of isolated pancreatic islets. PLoS ONE 2013, 8, e56799. [Google Scholar] [CrossRef]
- Jimbo, T.; Inagaki, A.; Imura, T.; Sekiguchi, S.; Nakamura, Y.; Fujimori, K.; Miyagawa, J.I.; Ohuchi, N.; Satomi, S.; Goto, M. A novel resting strategy for improving islet engraftment in the liver. Transplantation 2014, 97, 280–286. [Google Scholar] [CrossRef]
- Dendo, M.; Maeda, H.; Yamagata, Y.; Murayama, K.; Watanabe, K.; Imura, T.; Inagaki, A.; Igarashi, Y.; Katoh, Y.; Ebina, M.; et al. Synergistic effect of neutral protease and clostripain on rat pancreatic islet isolation. Transplantation 2015, 99, 1349–1355. [Google Scholar] [CrossRef]
- Pileggi, A.; Molano, R.D.; Ricordi, C.; Zahr, E.; Collins, J.; Valdes, R.; Inverardi, L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation 2006, 81, 1318–1324. [Google Scholar] [CrossRef]
- Kawakami, Y.; Iwata, H.; Gu, Y.J.; Miyamoto, M.; Murakami, Y.; Balamurugan, A.N.; Imamura, M.; Inoue, K. Successful subcutaneous pancreatic islet transplantation using an angiogenic growth factor-releasing device. Pancreas 2001, 23, 375–381. [Google Scholar] [CrossRef]
- Pepper, A.R.; Pawlick, R.; Gala-Lopez, B.; MacGillivary, A.; Mazzuca, D.M.; White, D.J.G.; Toleikis, P.M.; Shapiro, A.M.J. Diabetes Is Reversed in a Murine Model by Marginal Mass Syngeneic Islet Transplantation Using a Subcutaneous Cell Pouch Device. Transplantation 2015, 99, 2294–2300. [Google Scholar] [CrossRef]
- Golocheikine, A.; Tiriveedhi, V.; Angaswamy, N.; Benshoff, N.; Sabarinathan, R.; Mohanakumar, T. Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation. Transplantation 2010, 90, 725–731. [Google Scholar] [CrossRef]
- Gebe, J.A.; Preisinger, A.; Gooden, M.D.; D’Amico, L.A.; Vernon, R.B. Local, Controlled Release In Vivo of Vascular Endothelial Growth Factor Within a Subcutaneous Scaffolded Islet Implant Reduces Early Islet Necrosis and Improves Performance of the Graft. Cell Transplant. 2018, 27, 531–541. [Google Scholar] [CrossRef]
- Yap, W.T.; Salvay, D.M.; Silliman, M.A.; Zhang, X.; Bannon, Z.G.; Kaufman, D.B.; Lowe, W.L.; Shea, L.D. Collagen IV-modified scaffolds improve islet survival and function and reduce time to euglycemia. Tissue Eng. Part A 2013, 19, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Salvay, D.M.; Rives, C.B.; Zhang, X.; Chen, F.; Kaufman, D.B.; Lowe, W.L.J.; Shea, L.D. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation 2008, 85, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Merani, S.; Kin, T.; Shapiro, A.M. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 2015, 33, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Pawlick, R.; Bruni, A.; Gala-Lopez, B.; Wink, J.; Rafiei, Y.; Bral, M.; Abualhassan, N.; Shapiro, A.M.J. Harnessing the Foreign Body Reaction in Marginal Mass Device-less Subcutaneous Islet Transplantation in Mice. Transplantation 2016, 100, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Bruni, A.; Pawlick, R.L.; Gala-Lopez, B.; Rafiei, Y.; Wink, J.; Kin, T.; Shapiro, A.M.J. Long-term function and optimization of mouse and human islet transplantation in the subcutaneous device-less site. Islets 2016, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fumimoto, Y.; Matsuyama, A.; Komoda, H.; Okura, H.; Lee, C.M.; Nagao, A.; Nishida, T.; Ito, T.; Sawa, Y. Creation of a rich subcutaneous vascular network with implanted adipose tissue-derived stromal cells and adipose tissue enhances subcutaneous grafting of islets in diabetic mice. Tissue Eng. Part C Methods 2009, 15, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Brissova, M.; Fowler, M.; Wiebe, P.; Shostak, A.; Shiota, M.; Radhika, A.; Lin, P.C.; Gannon, M.; Powers, A.C. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes 2004, 53, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Nyqvist, D.; Kohler, M.; Wahlstedt, H.; Berggren, P.O. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 2005, 54, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Q.; Qi, L.; Dai, X.; Liu, H.; Wang, Y. Low levels of TGF-beta1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol. Med. Rep. 2016, 14, 1681–1692. [Google Scholar] [CrossRef]
- Olaniru, O.E.; Pingitore, A.; Giera, S.; Piao, X.; Castañera González, R.; Jones, P.M.; Persaud, S.J. The adhesion receptor GPR56 is activated by extracellular matrix collagen III to improve beta-cell function. Cell. Mol. Life Sci. 2018, 75, 4007–4019. [Google Scholar] [CrossRef]
- Duner, P.; Al-Amily, I.M.; Soni, A.; Asplund, O.; Safi, F.; Storm, P.; Groop, L.; Amisten, S.; Salehi, A. Adhesion G Protein-Coupled Receptor G1 (ADGRG1/GPR56) and Pancreatic beta-Cell Function. J. Clin. Endocrinol. Metab. 2016, 101, 4637–4645. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizui, T.; Inagaki, A.; Nakamura, Y.; Imura, T.; Uematsu, S.S.; Miyagi, S.; Kamei, T.; Unno, M.; Watanabe, K.; Goto, M. A Recombinant Peptide Device Combined with Adipose Tissue-Derived Stem Cells Enhances Subcutaneous Islet Engraftment. Cells 2024, 13, 499. https://doi.org/10.3390/cells13060499
Mizui T, Inagaki A, Nakamura Y, Imura T, Uematsu SS, Miyagi S, Kamei T, Unno M, Watanabe K, Goto M. A Recombinant Peptide Device Combined with Adipose Tissue-Derived Stem Cells Enhances Subcutaneous Islet Engraftment. Cells. 2024; 13(6):499. https://doi.org/10.3390/cells13060499
Chicago/Turabian StyleMizui, Takahiro, Akiko Inagaki, Yasuhiro Nakamura, Takehiro Imura, Satomi Suzuki Uematsu, Shigehito Miyagi, Takashi Kamei, Michiaki Unno, Kimiko Watanabe, and Masafumi Goto. 2024. "A Recombinant Peptide Device Combined with Adipose Tissue-Derived Stem Cells Enhances Subcutaneous Islet Engraftment" Cells 13, no. 6: 499. https://doi.org/10.3390/cells13060499