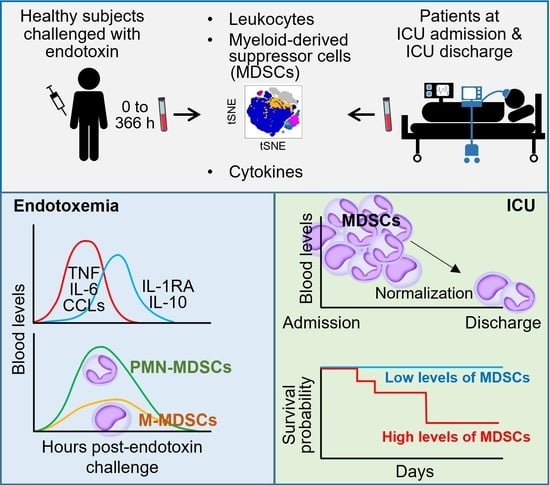
Myeloid-Derived Suppressor-like Cells as a Prognostic Marker in Critically Ill Patients: Insights from Experimental Endotoxemia and Intensive Care Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics, Subjects, and Study Design
2.2. Flow Cytometry
2.3. Measurement of Serum Mediators by Multiplex Bead Assay
2.4. Statistical Analysis
3. Results
3.1. MDSC-like Cells in Healthy Subjects Challenged with Endotoxin
3.2. MDSC-like Cells in Mechanically Ventilated ICU Patients
3.3. MDSC-like Cells in Relation with Cytokines and Growth Factors Levels in ICU Patients
3.4. MDSC-like Cells and Nosocomial Infections in ICU Patients
3.5. MDSC-like Cells and Outcome of ICU Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.K.; Pickkers, P.; van der Poll, T. Sepsis-Induced Immunosuppression. Annu. Rev. Physiol. 2022, 84, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Truck, J.; Roger, T. Editorial: The Immunology of Sepsis-Understanding Host Susceptibility, Pathogenesis of Disease, and Avenues for Future Treatment. Front. Immunol. 2020, 11, 1263. [Google Scholar] [CrossRef]
- Rubio, I.; Osuchowski, M.F.; Shankar-Hari, M.; Skirecki, T.; Winkler, M.S.; Lachmann, G.; La Rosee, P.; Monneret, G.; Venet, F.; Bauer, M.; et al. Current gaps in sepsis immunology: New opportunities for translational research. Lancet Infect. Dis. 2019, 19, e422–e436. [Google Scholar] [CrossRef]
- Ciarlo, E.; Savva, A.; Roger, T. Epigenetics in sepsis: Targeting histone deacetylases. Int. J. Antimicrob. Agents 2013, 42 (Suppl. S1), S8–S12. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.H.; Emmons, T.R.; Wong, J.T.; Alqassim, E.; Singel, K.L.; Mark, J.; Smith, B.E.; Tario, J.D.; Eng, K.H.; Moysich, K.B.; et al. Quantification of Early-Stage Myeloid-Derived Suppressor Cells in Cancer Requires Excluding Basophils. Cancer Immunol. Res. 2020, 8, 819–828. [Google Scholar] [CrossRef] [PubMed]
- De Zuani, M.; Hortova-Kohoutkova, M.; Andrejcinova, I.; Tomaskova, V.; Sramek, V.; Helan, M.; Fric, J. Human myeloid-derived suppressor cell expansion during sepsis is revealed by unsupervised clustering of flow cytometric data. Eur. J. Immunol. 2021, 51, 1785–1791. [Google Scholar] [CrossRef]
- Schrijver, I.T.; Theroude, C.; Roger, T. Myeloid-Derived Suppressor Cells in Sepsis. Front. Immunol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.T.; Gabrilovich, D.I.; Sansom, O.J.; Campbell, A.D.; Morton, J.P. Therapeutic targeting of tumour myeloid cells. Nat. Rev. Cancer 2023, 23, 216–237. [Google Scholar] [CrossRef]
- Uhel, F.; Azzaoui, I.; Gregoire, M.; Pangault, C.; Dulong, J.; Tadie, J.M.; Gacouin, A.; Camus, C.; Cynober, L.; Fest, T.; et al. Early Expansion of Circulating Granulocytic Myeloid-derived Suppressor Cells Predicts Development of Nosocomial Infections in Patients with Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 315–327. [Google Scholar] [CrossRef]
- Waeckel, L.; Venet, F.; Gossez, M.; Monard, C.; Rimmele, T.; Monneret, G. Delayed persistence of elevated monocytic MDSC associates with deleterious outcomes in septic shock: A retrospective cohort study. Crit. Care 2020, 24, 132. [Google Scholar] [CrossRef]
- Mathias, B.; Delmas, A.L.; Ozrazgat-Baslanti, T.; Vanzant, E.L.; Szpila, B.E.; Mohr, A.M.; Moore, F.A.; Brakenridge, S.C.; Brumback, B.A.; Moldawer, L.L.; et al. Human Myeloid-derived Suppressor Cells are Associated With Chronic Immune Suppression After Severe Sepsis/Septic Shock. Ann. Surg. 2017, 265, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Darden, D.B.; Bacher, R.; Brusko, M.A.; Knight, P.; Hawkins, R.B.; Cox, M.C.; Dirain, M.L.; Ungaro, R.; Nacionales, D.C.; Rincon, J.C.; et al. Single-Cell RNA-seq of Human Myeloid-Derived Suppressor Cells in Late Sepsis Reveals Multiple Subsets With Unique Transcriptional Responses: A Pilot Study. Shock 2021, 55, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, I.T.; Karakike, E.; Theroude, C.; Baumgartner, P.; Harari, A.; Giamarellos-Bourboulis, E.J.; Calandra, T.; Roger, T. High levels of monocytic myeloid-derived suppressor cells are associated with favorable outcome in patients with pneumonia and sepsis with multi-organ failure. Intensive Care Med. Exp. 2022, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Gentile, L.F.; Mathias, B.J.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017, 45, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Rincon, J.C.; Efron, P.A.; Moldawer, L.L. Immunopathology of chronic critical illness in sepsis survivors: Role of abnormal myelopoiesis. J. Leukoc. Biol. 2022, 112, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Sirivongrangson, P.; Kulvichit, W.; Payungporn, S.; Pisitkun, T.; Chindamporn, A.; Peerapornratana, S.; Pisitkun, P.; Chitcharoen, S.; Sawaswong, V.; Worasilchai, N.; et al. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med. Exp. 2020, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Antonakos, N.; Gilbert, C.; Theroude, C.; Schrijver, I.T.; Roger, T. Modes of action and diagnostic value of miRNAs in sepsis. Front. Immunol. 2022, 13, 951798. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis-a narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef]
- Povoa, P.; Coelho, L.; Dal-Pizzol, F.; Ferrer, R.; Huttner, A.; Morris, A.C.; Nobre, V.; Ramirez, P.; Rouze, A.; Salluh, J.; et al. How to use biomarkers of infection or sepsis at the bedside: Guide to clinicians. Intensive Care Med. 2023, 49, 142–153. [Google Scholar] [CrossRef]
- Van Engelen, T.S.R.; Wiersinga, W.J.; Scicluna, B.P.; van der Poll, T. Biomarkers in Sepsis. Crit. Care Clin. 2018, 34, 139–152. [Google Scholar] [CrossRef]
- Kox, M.; de Kleijn, S.; Pompe, J.C.; Ramakers, B.P.; Netea, M.G.; van der Hoeven, J.G.; Hoedemaekers, C.W.; Pickkers, P. Differential ex vivo and in vivo endotoxin tolerance kinetics following human endotoxemia. Crit. Care Med. 2011, 39, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Herderschee, J.; Heinonen, T.; Fenwick, C.; Schrijver, I.T.; Ohmiti, K.; Moradpour, D.; Cavassini, M.; Pantaleo, G.; Roger, T.; Calandra, T.; et al. High-dimensional immune phenotyping of blood cells by mass cytometry in patients infected with hepatitis C virus. Clin. Microbiol. Infect. 2022, 28, 611.e1–611.e7. [Google Scholar] [CrossRef]
- Schrijver, I.T.; Theroude, C.; Antonakos, N.; Regina, J.; Le Roy, D.; Bart, P.A.; Chiche, J.D.; Perreau, M.; Pantaleo, G.; Calandra, T.; et al. COVID-19 rapidly increases MDSCs and prolongs innate immune dysfunctions. Eur. J. Immunol. 2022, 52, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Perreau, M.; Suffiotti, M.; Marques-Vidal, P.; Wiedemann, A.; Levy, Y.; Laouenan, C.; Ghosn, J.; Fenwick, C.; Comte, D.; Roger, T.; et al. The cytokines HGF and CXCL13 predict the severity and the mortality in COVID-19 patients. Nat. Commun. 2021, 12, 4888. [Google Scholar] [CrossRef]
- Kan, T.; Feldman, E.; Timaner, M.; Raviv, Z.; Shen-Orr, S.; Aronheim, A.; Shaked, Y. IL-31 induces antitumor immunity in breast carcinoma. J. Immunother. Cancer 2020, 8, e001010. [Google Scholar] [CrossRef]
- Bonavia, A.S.; Samuelsen, A.; Luthy, J.; Halstead, E.S. Integrated machine learning approaches for flow cytometric quantification of myeloid-derived suppressor cells in acute sepsis. Front. Immunol. 2022, 13, 1007016. [Google Scholar] [CrossRef] [PubMed]
- Janols, H.; Bergenfelz, C.; Allaoui, R.; Larsson, A.M.; Ryden, L.; Bjornsson, S.; Janciauskiene, S.; Wullt, M.; Bredberg, A.; Leandersson, K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J. Leukoc. Biol. 2014, 96, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Patera, A.C.; Drewry, A.M.; Chang, K.; Beiter, E.R.; Osborne, D.; Hotchkiss, R.S. Frontline Science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J. Leukoc. Biol. 2016, 100, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Gey, A.; Tadie, J.M.; Caumont-Prim, A.; Hauw-Berlemont, C.; Cynober, L.; Fagon, J.Y.; Terme, M.; Diehl, J.L.; Delclaux, C.; Tartour, E. Granulocytic myeloid-derived suppressor cells inversely correlate with plasma arginine and overall survival in critically ill patients. Clin. Exp. Immunol. 2015, 180, 280–288. [Google Scholar] [CrossRef]
- Cassetta, L.; Bruderek, K.; Skrzeczynska-Moncznik, J.; Osiecka, O.; Hu, X.; Rundgren, I.M.; Lin, A.; Santegoets, K.; Horzum, U.; Godinho-Santos, A.; et al. Differential expansion of circulating human MDSC subsets in patients with cancer, infection and inflammation. J. Immunother. Cancer 2020, 8, e001223. [Google Scholar] [CrossRef]
- Condamine, T.; Dominguez, G.A.; Youn, J.I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef]
- Coudereau, R.; Waeckel, L.; Cour, M.; Rimmele, T.; Pescarmona, R.; Fabri, A.; Jallades, L.; Yonis, H.; Gossez, M.; Lukaszewicz, A.C.; et al. Emergence of immunosuppressive LOX-1+ PMN-MDSC in septic shock and severe COVID-19 patients with acute respiratory distress syndrome. J. Leukoc. Biol. 2021, 111, 489–496. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, X.; Zhang, J.; Jiang, X.; Wang, J.; Li, Y.; Li, X.; Shen, G.; Peng, J.; Zheng, P.; et al. CD300ld on neutrophils is required for tumour-driven immune suppression. Nature 2023, 621, 830–839. [Google Scholar] [CrossRef]
- Van Vlerken-Ysla, L.; Tyurina, Y.Y.; Kagan, V.E.; Gabrilovich, D.I. Functional states of myeloid cells in cancer. Cancer Cell 2023, 41, 490–504. [Google Scholar] [CrossRef]
- Palomino-Segura, M.; Sicilia, J.; Ballesteros, I.; Hidalgo, A. Strategies of neutrophil diversification. Nat. Immunol. 2023, 24, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.J.; Allcock, A.; Ferreira, R.C.; Cano-Gamez, E.; Smee, M.; Burnham, K.L.; Zurke, Y.X.; Research, O.E.M.; McKechnie, S.; Mentzer, A.J.; et al. Neutrophils and emergency granulopoiesis drive immune suppression and an extreme response endotype during sepsis. Nat. Immunol. 2023, 24, 767–779. [Google Scholar] [CrossRef]
- Tak, T.; van Groenendael, R.; Pickkers, P.; Koenderman, L. Monocyte Subsets Are Differentially Lost from the Circulation during Acute Inflammation Induced by Human Experimental Endotoxemia. J. Innate Immun. 2017, 9, 464–474. [Google Scholar] [CrossRef]
- Dong, G.; Si, C.; Zhang, Q.; Yan, F.; Li, C.; Zhang, H.; Ma, Q.; Dai, J.; Li, Z.; Shi, H.; et al. Autophagy regulates accumulation and functional activity of granulocytic myeloid-derived suppressor cells via STAT3 signaling in endotoxin shock. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Jiang, L.; Xu, X.; Zhu, F.; Zhang, S.; Wu, X.; Liu, Z.; Yang, X.; Li, S. STAT4 knockout protects LPS-induced lung injury by increasing of MDSC and promoting of macrophage differentiation. Respir. Physiol. Neurobiol. 2016, 223, 16–22. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Dong, G.; Yan, F.; Zhang, J.; Shi, H.; Ning, Z.; Gao, M.; Cheng, D.; Ma, Q.; et al. Toll-like Receptor 4 Inhibitor TAK-242 Improves Fulminant Hepatitis by Regulating Accumulation of Myeloid-Derived Suppressor Cell. Inflammation 2021, 44, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Brudecki, L.; Ferguson, D.A.; McCall, C.E.; El Gazzar, M. Myeloid-derived suppressor cells evolve during sepsis and can enhance or attenuate the systemic inflammatory response. Infect. Immun. 2012, 80, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Derive, M.; Bouazza, Y.; Alauzet, C.; Gibot, S. Myeloid-derived suppressor cells control microbial sepsis. Intensive Care Med. 2012, 38, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Romaschin, A.D.; Klein, D.J.; Marshall, J.C. Bench-to-bedside review: Clinical experience with the endotoxin activity assay. Crit. Care 2012, 16, 248. [Google Scholar] [CrossRef] [PubMed]
- Elbim, C.; Hakim, J.; Gougerot-Pocidalo, M.A. Heterogeneity in Lewis-X and sialyl-Lewis-X antigen expression on monocytes in whole blood: Relation to stimulus-induced oxidative burst. Am. J. Pathol. 1998, 152, 1081–1090. [Google Scholar]
- Pinto, L.A.; Trivett, M.T.; Wallace, D.; Higgins, J.; Baseler, M.; Terabe, M.; Belyakov, I.M.; Berzofsky, J.A.; Hildesheim, A. Fixation and cryopreservation of whole blood and isolated mononuclear cells: Influence of different procedures on lymphocyte subset analysis by flow cytometry. Cytometry B Clin. Cytom. 2005, 63, 47–55. [Google Scholar] [CrossRef]
- Paredes, R.M.; Tadaki, D.K.; Sooter, A.; Gamboni, F.; Sheppard, F. Cryopreservation of human whole blood allows immunophenotyping by flow cytometry up to 30days after cell isolation. J. Immunol. Methods 2018, 452, 32–38. [Google Scholar] [CrossRef]





| Endotoxin Study | ICU Study | ||
|---|---|---|---|
| Baseline | ICU Survivor | ICU Non-Survivor | |
| Number of subjects/patients | 8 | 29 | 4 |
| Gender, male | 8 (100%) * | 15 (52%) | 3 (75%) |
| Age (years) | 23.5 [22–27] * | 65 [52–68] | 63 [53–67] |
| Severity of illness at admission: | |||
| Mechanical ventilation | - | 29 (100%) | 4 (100%) |
| APACHE II score | - | 19 [16–23] | 20.5 [18.5–22.3] |
| SOFA score | - | 12 [10–14] | 11.5 [7.8–15.5] |
| Developed a secondary infection | - | 23 (79%) | 2 (50%) |
| Type of secondary infection: | |||
| VAP/HAP | - | 14 (48%) | 1 (25%) |
| HAI | - | 9 (31%) | 1 (25%) |
| ICU stay (days) | - | 8.5 [6.00–16.25] | 7 [5.50–9.25] |
| Leukocytes (× 109/L) | 4.7 [4.5–5.6] | 8.4 [5.6–9.8] | 6.0 [4.7–7.3] |
| CRP (mg/L) | - | 168 [122–281] | 103, 86 † |
| Lactate (mmol/L) | - | 1.1 [0.8–1.7] | 0.8, 0.6 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schrijver, I.T.; Herderschee, J.; Théroude, C.; Kritikos, A.; Leijte, G.; Le Roy, D.; Brochut, M.; Chiche, J.-D.; Perreau, M.; Pantaleo, G.; et al. Myeloid-Derived Suppressor-like Cells as a Prognostic Marker in Critically Ill Patients: Insights from Experimental Endotoxemia and Intensive Care Patients. Cells 2024, 13, 314. https://doi.org/10.3390/cells13040314
Schrijver IT, Herderschee J, Théroude C, Kritikos A, Leijte G, Le Roy D, Brochut M, Chiche J-D, Perreau M, Pantaleo G, et al. Myeloid-Derived Suppressor-like Cells as a Prognostic Marker in Critically Ill Patients: Insights from Experimental Endotoxemia and Intensive Care Patients. Cells. 2024; 13(4):314. https://doi.org/10.3390/cells13040314
Chicago/Turabian StyleSchrijver, Irene T., Jacobus Herderschee, Charlotte Théroude, Antonios Kritikos, Guus Leijte, Didier Le Roy, Maelick Brochut, Jean-Daniel Chiche, Matthieu Perreau, Giuseppe Pantaleo, and et al. 2024. "Myeloid-Derived Suppressor-like Cells as a Prognostic Marker in Critically Ill Patients: Insights from Experimental Endotoxemia and Intensive Care Patients" Cells 13, no. 4: 314. https://doi.org/10.3390/cells13040314