Metabolic Disruption Induced by mTOR Signaling Pathway Inhibition in Regulatory T-Cell Expansion for Clinical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Compounds
2.2. Purification and Activation of Primary Human T cells
2.3. Determination of the Minimal Effective Dose of AZD8055
2.4. Proliferation Assay
2.5. Cell Viability and Functional Assays
2.6. Cell Surface, Intracellular Staining and Flow Cytometry Analysis
2.7. Mitochondrial Integrity
2.8. Autophagy
2.9. Mitochondrial Unfolded Protein Response (mitoUPR) Cell Stress Response Pathway
2.10. Metabolic Characterization
2.11. Statistics
3. Results
3.1. Isolation and Culture of CD4+CD25+ Treg Cells
3.2. Signaling
3.3. Proliferation and Cell Viability
3.4. Suppressive Function
3.5. Mitochondrial Morphology and Function
3.6. Autophagic Response
3.7. mitoUPR Changes
3.8. Cell Metabolism and Bioenergetics
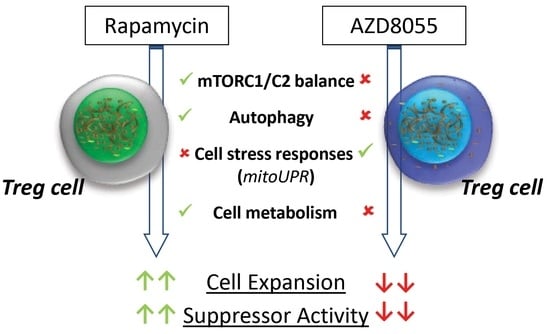
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tang, Q.; Henriksen, K.J.; Bi, M.; Finger, E.B.; Szot, G.; Ye, J.; Masteller, E.L.; McDevitt, H.; Bonyhadi, M.; Bluestone, J.A. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Scalapino, K.J.; Tang, Q.; Bluestone, J.A.; Bonyhadi, M.L.; Daikh, D.I. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol. 2006, 177, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Trenado, A.; Vasey, D.; Klatzmann, D.; Salomon, B.L. CD4(+)CD25(+) immunoregulatory T Cells: New therapeutics for graft-versus-host disease. J. Exp. Med. 2002, 196, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Ermann, J.; Edinger, M.; Fathman, C.G.; Strober, S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002, 196, 389–399. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Miller, J.S.; McKenna, D.H.; Hippen, K.L.; DeFor, T.E.; Sumstad, D.; Curtsinger, J.; Verneris, M.R.; MacMillan, M.L.; Levine, B.L.; et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: Kinetics, toxicity profile, and clinical effect. Blood 2016, 127, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- Martelli, M.F.; Di Ianni, M.; Ruggeri, L.; Falzetti, F.; Carotti, A.; Terenzi, A.; Pierini, A.; Massei, M.S.; Amico, L.; Urbani, E.; et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 2014, 124, 638–644. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Kole, T.P.; Zheng, Y.; Zarek, P.E.; Matthews, K.L.; Xiao, B.; Worley, P.F.; Kozma, S.C.; Powell, J.D. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 2009, 30, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Long, L.; Zhou, P.; Chapman, N.M.; Chi, H. mTOR signaling at the crossroads of environmental signals and T-cell fate decisions. Immunol. Rev. 2020, 295, 15–38. [Google Scholar] [CrossRef]
- Battaglia, M.; Stabilini, A.; Migliavacca, B.; Horejs-Hoeck, J.; Kaupper, T.; Roncarolo, M.-G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. Baltim. Md 1950 2006, 177, 8338–8347. [Google Scholar] [CrossRef] [Green Version]
- Turnquist, H.R.; Raimondi, G.; Zahorchak, A.F.; Fischer, R.T.; Wang, Z.; Thomson, A.W. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. Baltim. Md 1950 2007, 178, 7018–7031. [Google Scholar] [CrossRef] [Green Version]
- Strauss, L.; Czystowska, M.; Szajnik, M.; Mandapathil, M.; Whiteside, T.L. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS ONE 2009, 4, e5994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiser, R.; Leveson-Gower, D.B.; Zambricki, E.A.; Kambham, N.; Beilhack, A.; Loh, J.; Hou, J.-Z.; Negrin, R.S. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood 2008, 111, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valmori, D.; Tosello, V.; Souleimanian, N.E.; Godefroy, E.; Scotto, L.; Wang, Y.; Ayyoub, M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol. Baltim. Md 1950 2006, 177, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Abanni, L.; Calì, G.; Porcellini, A.; Carbone, F.; Fontana, S.; Horvath, T.L.; La Cava, A.; et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity 2010, 33, 929–941. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Yang, K.; Cloer, C.; Neale, G.; Vogel, P.; Chi, H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature 2013, 499, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gedaly, R.; De Stefano, F.; Turcios, L.; Hill, M.; Hidalgo, G.; Mitov, M.I.; Alstott, M.C.; Butterfield, D.A.; Mitchell, H.C.; Hart, J.; et al. mTOR Inhibitor Everolimus in Regulatory T Cell Expansion for Clinical Application in Transplantation. Transplantation 2019, 103, 705–715. [Google Scholar] [CrossRef]
- Vilchez, V.; Turcios, L.; Butterfield, D.A.; Mitov, M.I.; Coquillard, C.L.; Brandon, J.A.; Cornea, V.; Gedaly, R.; Marti, F. Evidence of the immunomodulatory role of dual PI3K/mTOR inhibitors in transplantation: An experimental study in mice. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2017, 30, 1061–1074. [Google Scholar] [CrossRef] [Green Version]
- Gedaly, R.; Cornea, V.; Turcios, L.; Edmisson, J.S.; Harris, D.D.; Watt, D.S.; Chapelin, F.; Khurana, A.; Mei, X.; Liu, C.; et al. Anti-neoplastic sulfonamides alter the metabolic homeostasis and disrupt the suppressor activity of regulatory T cells. Sci. Rep. 2022, 12, 19112. [Google Scholar] [CrossRef]
- Ellis, G.I.; Zhi, L.; Akundi, R.; Büeler, H.; Marti, F. Mitochondrial and cytosolic roles of PINK1 shape induced regulatory T-cell development and function. Eur. J. Immunol. 2013, 43, 3355–3360. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewicz, M.; Ronowska, A.; Zieliński, M.; Jankowska-Kulawy, A.; Trzonkowski, P. T regulatory cells metabolism: The influence on functional properties and treatment potential. Front. Immunol. 2023, 14, 1122063. [Google Scholar] [CrossRef]
- Chresta, C.M.; Davies, B.R.; Hickson, I.; Harding, T.; Cosulich, S.; Critchlow, S.E.; Vincent, J.P.; Ellston, R.; Jones, D.; Sini, P.; et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010, 70, 288–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmond, R.J.; Filby, A.; Qureshi, I.; Caserta, S.; Zamoyska, R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 2009, 228, 9–22. [Google Scholar] [CrossRef]
- Claus, C.; Chey, S.; Heinrich, S.; Reins, M.; Richardt, B.; Pinkert, S.; Fechner, H.; Gaunitz, F.; Schäfer, I.; Seibel, P.; et al. Involvement of p32 and Microtubules in Alteration of Mitochondrial Functions by Rubella Virus. J. Virol. 2011, 85, 3881–3892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harder, L.M.; Bunkenborg, J.; Andersen, J.S. Inducing autophagy: A comparative phosphoproteomic study of the cellular response to ammonia and rapamycin. Autophagy 2014, 10, 339–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacarelli, T.; Azar, A.; Altinok, O.; Orynbayeva, Z.; Sell, C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. GeroScience 2018, 40, 243–256. [Google Scholar] [CrossRef] [PubMed]
- van der Windt, G.J.W.; Pearce, E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012, 249, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckl, E.-M.; Ziegemann, O.; Krumwiede, L.; Fessler, E.; Jae, L.T. Sensing, signaling and surviving mitochondrial stress. Cell. Mol. Life Sci. CMLS 2021, 78, 5925–5951. [Google Scholar] [CrossRef]
- Li, R.; Jen, N.; Wu, L.; Lee, J.; Fang, K.; Quigley, K.; Lee, K.; Wang, S.; Zhou, B.; Vergnes, L.; et al. Disturbed Flow Induces Autophagy, but Impairs Autophagic Flux to Perturb Mitochondrial Homeostasis. Antioxid. Redox Signal. 2015, 23, 1207–1219. [Google Scholar] [CrossRef] [Green Version]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. Baltim. Md 1950 2011, 186, 3299–3303. [Google Scholar] [CrossRef] [Green Version]
- Beier, U.H.; Angelin, A.; Akimova, T.; Wang, L.; Liu, Y.; Xiao, H.; Koike, M.A.; Hancock, S.A.; Bhatti, T.R.; Han, R.; et al. Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB J. 2015, 29, 2315–2326. [Google Scholar] [CrossRef] [Green Version]
- Shpilka, T.; Haynes, C.M. The mitochondrial UPR: Mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 2018, 19, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.-H.; Jones, R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Green, D.R. Metabolic reprogramming and metabolic dependency in T cells. Immunol. Rev. 2012, 249, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Target | Rapamycin | AZD8055 | |
|---|---|---|---|
| Signaling | mTORC1 | ↓ | ↓ |
| mTORC2 | ◯ | ↓ | |
| IL2-R pathway | ◯ | ↓ | |
| Cell function | Expansion | ↓ | ↓↓ |
| Suppressor Activity | ↑ | ↓ | |
| Mechanism of action | Mitochondrial stress | ◯ | ↑ |
| Autophagy | ↑ | ↓ | |
| mitoUPR | ◯/↑ | ↑↑ | |
| Cell bioenergetics | ◯ | ↓↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gedaly, R.; Orozco, G.; Ancheta, A.P.; Donoho, M.; Desai, S.N.; Chapelin, F.; Khurana, A.; Lewis, L.J.; Zhang, C.; Marti, F. Metabolic Disruption Induced by mTOR Signaling Pathway Inhibition in Regulatory T-Cell Expansion for Clinical Application. Cells 2023, 12, 2066. https://doi.org/10.3390/cells12162066
Gedaly R, Orozco G, Ancheta AP, Donoho M, Desai SN, Chapelin F, Khurana A, Lewis LJ, Zhang C, Marti F. Metabolic Disruption Induced by mTOR Signaling Pathway Inhibition in Regulatory T-Cell Expansion for Clinical Application. Cells. 2023; 12(16):2066. https://doi.org/10.3390/cells12162066
Chicago/Turabian StyleGedaly, Roberto, Gabriel Orozco, Alexandre P. Ancheta, Mackenzie Donoho, Siddharth N. Desai, Fanny Chapelin, Aman Khurana, Lillie J. Lewis, Cuiping Zhang, and Francesc Marti. 2023. "Metabolic Disruption Induced by mTOR Signaling Pathway Inhibition in Regulatory T-Cell Expansion for Clinical Application" Cells 12, no. 16: 2066. https://doi.org/10.3390/cells12162066