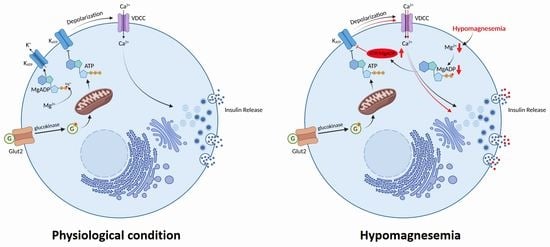
Chronic Mg2+ Deficiency Does Not Impair Insulin Secretion in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Islet Isolation and Determination of Insulin Secretion
2.2. Calcium Imaging
2.3. Calculation of Calcium Oscillation Frequency and Amplitude
2.4. Characterization of Glucose Homeostasis
2.5. Statistics
3. Results
3.1. Lowering Extracellular Mg2+ Concentrations Enhances GIIS in Murine Islets
3.2. Lowering Extracellular Mg2+ Concentrations Augments Ca2+ Oscillatory Amplitudes in Murine Islets
3.3. Plasma Levels of Insulin and GIIS Rise in Intestine-Specific Trpm6 KO Mice
3.4. Intestine-Specific Trpm6 KO Islets Exhibit an Increased Ca2+ Oscillation Amplitude
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alswat, K. Type 2 diabetes control and complications and their relation to serum magnesium level. Arch. Med. Sci. 2022, 18, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.-C.T.; Pham, P.-M.T.; Pham, S.V.; Miller, J.M.; Pham, P.-T.T. Hypomagnesemia in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2007, 2, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garfinkel, L.; Garfinkel, D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium 1985, 4, 60–72. [Google Scholar] [PubMed]
- Matschinsky, F.M. Regulation of pancreatic beta-cell glucokinase: From basics to therapeutics. Diabetes 2002, 51 (Suppl. S3), S394–S404. [Google Scholar] [CrossRef] [Green Version]
- Molnes, J.; Teigen, K.; Aukrust, I.; Bjørkhaug, L.; Søvik, O.; Flatmark, T.; Njølstad, P.R. Binding of ATP at the active site of human pancreatic glucokinase-nucleotide-induced conformational changes with possible implications for its kinetic cooperativity. FEBS J. 2011, 278, 2372–2386. [Google Scholar] [CrossRef] [Green Version]
- Storer, A.C.; Cornish-Bowden, A. Kinetics of rat liver glucokinase. Co-operative interactions with glucose at physiologically significant concentrations. Biochem. J. 1976, 159, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.; Ramracheya, R.; Bengtsson, M.; Zhang, Q.; Karanauskaite, J.; Partridge, C.; Johnson, P.R.; Rorsman, P. Voltage-gated ion channels in human pancreatic beta-cells: Electrophysiological characterization and role in insulin secretion. Diabetes 2008, 57, 1618–1628. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.L.; Hales, C.N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 1984, 311, 271–273. [Google Scholar] [CrossRef]
- Dunne, M.J.; Petersen, O.H. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986, 208, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Kakei, M.; Kelly, R.P.; Ashcroft, S.J.; Ashcroft, F.M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986, 208, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Gommers, L.M.M.; Hill, T.G.; Ashcroft, F.M.; De Baaij, J.H.F. Low extracellular magnesium does not impair glucose-stimulated insulin secretion. PLoS ONE 2019, 14, e0217925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gommers, L.M.M.; Hoenderop, J.G.J.; Bindels, R.J.M.; De Baaij, J.H.F. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.B.; Lu, Y.; Choate, K.A.; Velazquez, H.; Al-Sabban, E.; Praga, M.; Casari, G.; Bettinelli, A.; Colussi, G.; Rodriguez-Soriano, J.; et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 1999, 285, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Walder, R.Y.; Landau, D.; Meyer, P.; Shalev, H.; Tsolia, M.; Borochowitz, Z.; Boettger, M.B.; Beck, G.E.; Englehardt, R.K.; Carmi, R.; et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat. Genet. 2002, 31, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Arjona, F.; de Baaij, J.; Schlingmann, K.P.; Lameris, A.L.L.; Van Wijk, E.; Flik, G.; Regele, S.; Korenke, G.C.; Neophytou, B.; Rust, S.; et al. CCNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesemia. PLoS Genet. 2014, 10, e1004267. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Niemann Nejsum, L.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.C.M.; Droogmans, G.; Bindels, R.J.; Hoenderop, J.G. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Chubanov, V.; Ferioli, S.; Wisnowsky, A.; Simmons, D.G.; Leitzinger, C.; Einer, C.; Jonas, W.; Shymkiv, Y.; Bartsch, H.; Braun, A.; et al. Epithelial magnesium transport by TRPM6 is essential for prenatal development and adult survival. eLife 2016, 5, e20914. [Google Scholar] [CrossRef]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Khajavi, N.; Finan, B.; Kluth, O.; Müller, T.D.; Mergler, S.; Schulz, A.; Kleinau, G.; Scheerer, P.; Schürmann, A.; Gudermann, T.; et al. An incretin-based tri-agonist promotes superior insulin secretion from murine pancreatic islets via PLC activation. Cell. Signal. 2018, 51, 13–22. [Google Scholar] [CrossRef]
- Khajavi, N.; Beck, A.; Riçku, K.; Beyerle, P.; Jacob, K.; Syamsul, S.F.; Belkacemi, A.; Reinach, P.S.; Schreier, P.C.; Salah, H.; et al. TRPM7 kinase is required for insulin production and compensatory islet responses during obesity. JCI Insight 2023, 8, e163397. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152–1157. [Google Scholar] [CrossRef] [Green Version]
- ELDerawi, W.A.; Naser, I.A.; Taleb, M.H.; Abutair, A.S. The Effects of Oral Magnesium Supplementation on Glycemic Response among Type 2 Diabetes Patients. Nutrients 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamilian, M.; Mirhosseini, N.; Eslahi, M.; Bahmani, F.; Shokrpour, M.; Chamani, M.; Asemi, Z. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth 2019, 19, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Morán, M.; Guerrero-Romero, F. Insulin secretion is decreased in non-diabetic individuals with hypomagnesaemia. Diabetes Metab. Res. Rev. 2011, 27, 590–596. [Google Scholar] [CrossRef]
- Murakami, M.; Ishizuka, J.; Sumi, S.; Nickols, G.A.; Cooper, C.W.; Townsend, C.M.; Thompson, J.C. Role of extracellular magnesium in insulin secretion from rat insulinoma cells. Proc. Soc. Exp. Biol. Med. 1992, 200, 490–494. [Google Scholar] [CrossRef]
- Ishizuka, J.; Bold, R.J.; Townsend, C.M.; Thompson, J.C. In vitro relationship between magnesium and insulin secretion. Magnes. Res. 1994, 7, 17–22. [Google Scholar]
- Reis, M.A.; Latorraca, M.Q.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.; Velloso, L.A.; Reyes, F.G. Magnesium deficiency improves glucose homeostasis in the rat: Studies in vivo and in isolated islets in vitro. Br. J. Nutr. 2001, 85, 549–552. [Google Scholar] [CrossRef] [Green Version]
- Legrand, C.; Okitolonda, W.; Pottier, A.; Lederer, J.; Henquin, J. Glucose homeostasis in magnesium-deficient rats. Metabolism 1987, 36, 160–164. [Google Scholar] [CrossRef]
- Balon, T.W.; Gu, J.L.; Tokuyama, Y.; Jasman, A.P.; Nadler, J.L. Magnesium supplementation reduces development of diabetes in a rat model of spontaneous NIDDM. Am. J. Physiol. 1995, 269 Pt 1, E745–E752. [Google Scholar] [CrossRef]
- Ryazanova, L.V.; Dorovkov, M.V.; Ansari, A.; Ryazanov, A.G. Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J. Biol. Chem. 2004, 279, 3708–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Randell, E.W.; Mathews, M.; Gadag, V.; Zhang, H.; Sun, G. Relationship between serum magnesium values, lipids and anthropometric risk factors. Atherosclerosis 2008, 196, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Proks, P.; de Wet, H.; Ashcroft, F.M. Activation of the K(ATP) channel by Mg-nucleotide interaction with SUR1. J. Gen. Physiol. 2010, 136, 389–405. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Berlin, J.R. Channel phosphorylation and modulation of L-type Ca2+ currents by cytosolic Mg2+ concentration. Am. J. Physiol. Cell Physiol. 2006, 291, C83–C92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Berra-Romani, R.; Sinnegger-Brauns, M.J.; Striessnig, J.; Blaustein, M.P.; Matteson, D.R. Role of Cav1.2 L-type Ca2+ channels in vascular tone: Effects of nifedipine and Mg2+. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H415–H425. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, T.; Wang, Y.; Morishima, M.; Miyamoto, S.; Ono, K. Hypomagnesemic down-regulation of L-type Ca(2+) channel in cardiomyocyte as an arrhythmogenic substrate in rats. Pathophysiology 2015, 22, 87–93. [Google Scholar] [CrossRef]
- Gavin, J.R., III; Roth, J.; Neville, D.M., Jr.; De Meyts, P.; Buell, D.N. Insulin-dependent regulation of insulin receptor concentrations: A direct demonstration in cell culture. Proc. Natl. Acad. Sci. USA 1974, 71, 84–88. [Google Scholar] [CrossRef]
- Dasgupta, A.; Sarma, D.; Saikia, U.K. Hypomagnesemia in type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2012, 16, 1000–1003. [Google Scholar] [CrossRef]
- McNair, P.; Christensen, M.S.; Christiansen, C.; Madsbad, S.; Transbøl, I. Renal hypomagnesaemia in human diabetes mellitus: Its relation to glucose homeostasis. Eur. J. Clin. Investig. 1982, 12, 81–85. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khajavi, N.; Riçku, K.; Schreier, P.C.F.; Gentz, T.; Beyerle, P.; Cruz, E.; Breit, A.; Reinach, P.S.; Gudermann, T. Chronic Mg2+ Deficiency Does Not Impair Insulin Secretion in Mice. Cells 2023, 12, 1790. https://doi.org/10.3390/cells12131790
Khajavi N, Riçku K, Schreier PCF, Gentz T, Beyerle P, Cruz E, Breit A, Reinach PS, Gudermann T. Chronic Mg2+ Deficiency Does Not Impair Insulin Secretion in Mice. Cells. 2023; 12(13):1790. https://doi.org/10.3390/cells12131790
Chicago/Turabian StyleKhajavi, Noushafarin, Klea Riçku, Pascale C. F. Schreier, Tanja Gentz, Philipp Beyerle, Emmanuel Cruz, Andreas Breit, Peter S. Reinach, and Thomas Gudermann. 2023. "Chronic Mg2+ Deficiency Does Not Impair Insulin Secretion in Mice" Cells 12, no. 13: 1790. https://doi.org/10.3390/cells12131790