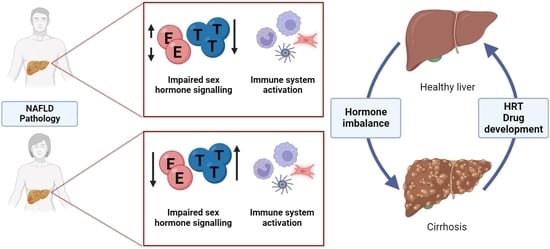
The Influence of Sex Hormones in Liver Function and Disease
Abstract
:1. Introduction
2. Sex Steroid Biosynthesis, Signaling and Regulation of Liver Function
2.1. Overview of Sex Hormone Biosynthesis
2.2. Circulating Hormones in Women
2.3. Circulating Hormones in Men
2.4. Receptor-Dependent Signaling by Estrogens and Androgens
2.5. Genomic Pathway
2.6. Non-Genomic Pathway
2.7. Ligand-Independent Signaling Pathway by Estrogens and Androgens
2.8. Estrogen Signaling in Liver
2.9. Androgen Signaling in Liver Pathology
| Animal | Sex | Modification | Phenotype | Reference |
|---|---|---|---|---|
| Mouse | Male | ArKO (aromatase deficient) in liver and muscle | Obese Hyperglycemia Insulin resistanceUpon E2 administration: recovery to WT phenotype | [39] |
| Mouse | Female | ArKO | 10-fold elevated testosterone compared to wild type (WT) | [40] |
| Mouse | Male | 5α-reductase1-KO (Srd5a1−/−) | Impaired testosterone to DHT conversion Obesity Hyperinsulinemia Hepatic steatosis Predisposition to hepatic fibrosis | [41,42] |
| Mouse | Female | MOER (only plasma membrane ERα) | Normal response to E2 in ERK and PI3K activation Abnormal reproductive tract, mammary gland, hormone secretion Obesity without functional nuclear ERα | [102,103] |
| Mouse | Female | ERKO (ERα knockout) and LERKO (liver-specific ERKO) | Higher body weight than WT Insulin resistance Higher leptin levels Fasting hyperinsulinaemia Hyperglycemia Altered hepatokine production | [73,74,95,98,104] |
| Mouse | Male | ERKO (ERα knockout) | Similar body weight to WT Fasting hyperinsulinemia Hyperglycemia | [73,74,104] |
| Mouse | Female | BERKO (ERβ knockout) | Normal body weight | [73] |
| Mouse | Female | GPER1-KO (Gpr30-LacZ) and high-fat diet supplementation | Young mice: Lower levels of HDL, higher risk of liver injury | [85] |
| Mouse | Male | GPER1-KO (Gpr30-LacZ) and high-fat diet supplementation | Young mice: No lipid profile changes, reduced expression of liver damage markers (ALAT, ASAT) Old mice: Weight gain, elevated triglyceride levels and cholesterol | [85,86] |
| Mouse | Male | ARKO (androgen receptor knockout) | Failure to develop male phenotype Reduced testes and serum testosterone levels Decreased fatty acid β-oxidation and PPARα expression Triglyceride accumulation Hepatic steatosis Insulin resistance Leptin resistance Risk of T2D | [76,115,121] |
| Mouse | Female | ARKO | Impaired ductal system within mammary glands Insulin resistance Hepatic steatosis | [121,122] |
| Rat Mouse | Female | OVX (ovariectomized) | Removes ovarian E2 Impaired glycogen synthesis Impaired TCA cycle Risk of MetS and NAFLD Altered hepatokine production | [106,123] [98] |
| Rat Mouse | Male | Castrated Testicular feminized | Disrupted testosterone production Impaired regulation of glucose transporters Elevated blood glucose levels Risk of T2D Increased hepatic lipid deposition | [120] [114] |
2.10. Hormone Replacement Therapies for NAFLD
3. The Role of the Immune System in Liver Biology and Metabolism
3.1. Immunity and NAFLD/NASH
3.2. Immunometabolism in NAFLD/NASH
4. Current Non-Alcoholic Fatty Liver Disease In Vitro Modeling Systems
4.1. Overview of Non-Alcoholic Fatty Liver Disease in Humans
4.2. Cell-Based Modeling of NAFLD
4.3. Genetically Defined Models of Liver Steatosis
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 17β-HSD | 17β-hydroxysteroid dehydrogenase |
| 3β-HSD | 3β-hydroxysteroid dehydrogenase D5-D4 isomerase |
| Acaca | acetyl-CoA carboxylase alpha |
| ALT | alanine transaminase |
| AR | androgen receptor |
| ARE | androgen response element |
| CLiP | chemically induced hepatic progenitor |
| CXCL10 | C-X-C motif ligand 10 |
| CYP11A1 | cytochrome P450 cholesterol side-chain cleavage enzyme |
| CYP17A1 | cytochrome P450 17α-hydroxylase |
| CYP19A1 | cytochrome P450 aromatase |
| CYP1A2 | cytochrome P450 1A2 |
| CYP3A4/5 | cytochrome P450 3A4/5 |
| CYP450 | cytochrome P450 family |
| CYP4A1 | cytochrome P450 4A1 |
| DAMPs | damage-associated molecular patterns |
| DHEA | dehydroepiandrosterone |
| DHEA-S | dehydroepiandrosterone sulphate |
| DHT | dihydrotestosterone |
| E1 | estrone |
| E2 | estradiol |
| E3 | estriol |
| E4 | estetrol |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| ERE | estrogen response element |
| ERα | estrogen receptor alpha |
| ERβ | estrogen receptor beta |
| ETC | electron transport chain |
| FFAs | free fatty acids |
| GLUT | glucose transporters |
| GPER1 | G protein-coupled estrogen receptor 1 |
| HCC | hepatocellular carcinoma |
| HDL | high-density lipoprotein |
| HIF-1α | hypoxia-inducible factor 1α |
| HPC | hepatic progenitor cell |
| HRE | hormone response element |
| HRT | hormone replacement therapy |
| HSC | hepatic stellate cell |
| IL | interleukin |
| LDL | low-density lipoprotein |
| LPS | lipopolysaccharide |
| MetS | metabolic syndrome |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| OXPHOS | oxidative phosphorylation |
| PHH | primary human hepatocyte |
| PPAR | peroxisome proliferator-activated receptor |
| PPP | pentose phosphate pathway |
| PSC | pluripotent stem cell |
| ROR | retinoid orphan receptor |
| ROS | reactive oxygen species |
| Scd1 | stearoyl-CoA desaturase 1 |
| SHBG | sex hormone-binding globulin |
| T | testosterone |
| T2D | type 2 diabetes |
| TCA | tricarboxylic acid |
| TH | T helper cell |
| TLR4 | toll-like receptor 4 |
| TNFα | tumour necrosis factor alpha |
| TNFR1 | TNFα receptor |
References
- Szkolnicka, D.; Hay, D. Liver stem cells. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J., Atala, A., Eds.; Elsevier: Amsterdam, The Netherland, 2020; Chapter 40; pp. 825–845. [Google Scholar]
- Zorn, A.M. Liver Development StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008. [Google Scholar]
- Sadri, A.-R.; Jeschke, M.G.; Amini-Nik, S. Advances in Liver Regeneration: Revisiting Hepatic Stem/Progenitor Cells and Their Origin. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyajima, A.; Tanaka, M.; Itoh, T. Stem/Progenitor Cells in Liver Development, Homeostasis, Regeneration, and Reprogramming. Cell Stem Cell 2014, 14, 561–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidi, H.; Luu, N.-T.; Alwahsh, S.M.; Ginai, M.; Alhaque, S.; Dong, H.; Tomaz, R.A.; Vernay, B.; Vigneswara, V.; Hallett, J.M.; et al. 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch. Toxicol. 2018, 92, 3117–3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, R.D.; Mitry, R.R.; Dhawan, A. Hepatocyte Transplantation for Metabolic Liver Disease: UK Experience. J. R. Soc. Med. 2005, 98, 341–345. [Google Scholar] [CrossRef]
- Alwahsh, S.M.; Rashidi, H.; Hay, D.C. Liver cell therapy: Is this the end of the beginning? Cell. Mol. Life Sci. 2018, 75, 1307–1324. [Google Scholar] [CrossRef] [Green Version]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef] [Green Version]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef]
- Ramachandran, P.; Iredale, J.P. Liver fibrosis: A bidirectional model of fibrogenesis and resolution. Qjm Int. J. Med. 2012, 105, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef]
- Iredale, J. Defining therapeutic targets for liver fibrosis: Exploiting the biology of inflammation and repair. Pharmacol. Res. 2008, 58, 129–136. [Google Scholar] [CrossRef]
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Grønbæk, H. Bariatric surgery in patients with non-alcoholic fatty liver disease—From pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Carulli, L.; Lonardo, A.; Lombardini, S.; Marchesini, G.; Loria, P. Gender, fatty liver and GGT. Hepatology 2006, 44, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef] [Green Version]
- Lyall, M.; Cartier, J.; Thomson, J.P.; Cameron, K.; Ripolles, J.M.; O’Duibhir, E.; Szkolnicka, D.; Villarin, B.L.; Wang, Y.; Blanco, G.R.; et al. Modelling non-alcoholic fatty liver disease in human hepatocyte-like cells. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170362. [Google Scholar] [CrossRef] [Green Version]
- Szkolnicka, D.; Hay, D.C. Concise Review: Advances in Generating Hepatocytes from Pluripotent Stem Cells for Translational Medicine. Stem Cells 2016, 34, 1421–1426. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.H.; Hales, D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004, 25, 947–970. [Google Scholar] [CrossRef] [Green Version]
- Wierman, M.E. Sex steroid effects at target tissues: Mechanisms of action. Adv. Physiol. Educ. 2007, 31, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Loggie, B.W.; Nawaz, Z. The roles of sex steroid receptor coregulators in cancer. Mol. Cancer 2002, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Kuan, K.K.W.; Saunders, P.T.K. Female Reproductive Systems: Hormone Dependence and Receptor Expression. Adv. Exp. Med. Biol. 2022, 1390, 21–39. [Google Scholar] [CrossRef]
- Gibson, D.; Simitsidellis, I.; Collins, F.; Saunders, P.T.K. Androgens, oestrogens and endometrium: A fine balance between perfection and pathology. J. Endocrinol. 2020, 246, R75–R93. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Au, C.C.; Benito-Martin, A.; Ladumor, H.; Oshchepkova, S.; Moges, R.; Brown, K.A. Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J. Steroid Biochem. Mol. Biol. 2019, 189, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Nwachukwu, J.C.; Min, C.K.; Njeri, J.W.; Srinivasan, S.; Rangarajan, E.S.; Nettles, C.C.; Guillen, V.S.; Ziegler, Y.; Yan, S.; et al. Dual-mechanism estrogen receptor inhibitors. Proc. Natl. Acad. Sci. USA 2021, 118, e2101657118. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef] [PubMed]
- Eacker, S.M.; Agrawal, N.; Qian, K.; Dichek, H.L.; Gong, E.-Y.; Lee, K.; Braun, R.E. Hormonal Regulation of Testicular Steroid and Cholesterol Homeostasis. Mol. Endocrinol. 2008, 22, 623–635. [Google Scholar] [CrossRef] [Green Version]
- Dieni, C.V.; Contemori, S.; Biscarini, A.; Panichi, R. De Novo Synthesized Estradiol: A Role in Modulating the Cerebellar Function. Int. J. Mol. Sci. 2020, 21, 3316. [Google Scholar] [CrossRef]
- McEwan, I.J.; Brinkmann, A.O. Androgen Physiology: Receptor and Metabolic Disorders. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Li, L.; Zirkin, B.R.; Papadopoulos, V. Leydig Cell Androgen Synthesis. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 215–221. [Google Scholar]
- Zhu, Y.S.; Sun, G.H. 5α-Reductase Isozymes in the Prostate. J. Med. Sci. 2005, 25, 1–12. [Google Scholar]
- Stoffel-Wagner, B.; Watzka, M.; Steckelbroeck, S.; Schramm, J.; Bidlingmaier, J.F.; Klingmuller, D. Expression of 17beta-hydroxysteroid dehydrogenase types 1, 2, 3 and 4 in the human temporal lobe. J. Endocrinol. 1999, 160, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Chai, Z.; Brereton, P.; Suzuki, T.; Sasano, H.; Obeyesekere, V.; Escher, G.; Saffery, R.; Fuller, P.; Enriquez, C.; Krozowski, Z. 17β-Hydroxysteroid Dehydrogenase Type XI Localizes to Human Steroidogenic Cells. Endocrinology 2003, 144, 2084–2091. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, J.; Li, H.; Dong, B.; Jiang, J.; Liu, N.; Tan, J.; Wang, X.; Lei, L.; Li, H.; et al. Down-Regulating the High Level of 17-Beta-Hydroxysteroid Dehydrogenase 13 Plays a Therapeutic Role for Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 5544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-F.; Wang, S.-G.; Zhao, Z.-Y.; Li, W.-L. AKR1C1-3, notably AKR1C3, are distinct biomarkers for liver cancer diagnosis and prognosis: Database mining in malignancies. Oncol. Lett. 2019, 18, 4515–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitling, R.; Krazeisen, A.; Möller, G.; Adamski, J. 17β-hydroxysteroid dehydrogenase type 7—An ancient 3-ketosteroid reductase of cholesterogenesis. Mol. Cell. Endocrinol. 2001, 171, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Hata, S.; Miki, Y.; Saito, R.; Ishida, K.; Watanabe, M.; Sasano, H. Aromatase in human liver and its diseases. Cancer Med. 2013, 2, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Toda, A.; Ono, M.; Saibara, T. Lack of 17β-estradiol reduces sensitivity to insulin in the liver and muscle of male mice. Heliyon 2018, 4, e00772. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.R.; Graves, K.H.; Parlow, A.F.; Simpson, E.R. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. USA 1998, 95, 6965–6970. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, D.E.; Barat, P.; Di Rollo, E.M.; Rees, G.A.; Weldin, B.A.; Rog-Zielinska, E.A.; MacFarlane, D.P.; Walker, B.R.; Andrew, R. 5α-Reductase Type 1 Deficiency or Inhibition Predisposes to Insulin Resistance, Hepatic Steatosis, and Liver Fibrosis in Rodents. Diabetes 2014, 64, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Dowman, J.K.; Hopkins, L.J.; Reynolds, G.M.; Armstrong, M.J.; Nasiri, M.; Nikolaou, N.; Van Houten, E.L.A.F.; Visser, J.A.; Morgan, S.A.; Lavery, G.G.; et al. Loss of 5α-Reductase Type 1 Accelerates the Development of Hepatic Steatosis but Protects Against Hepatocellular Carcinoma in Male Mice. Endocrinology 2013, 154, 4536–4547. [Google Scholar] [CrossRef]
- Farkas, S.; Szabó, A.; Hegyi, A.E.; Török, B.; Fazekas, C.L.; Ernszt, D.; Kovács, T.; Zelena, D. Estradiol and Estrogen-like Alternative Therapies in Use: The Importance of the Selective and Non-Classical Actions. Biomedicines 2022, 10, 861. [Google Scholar] [CrossRef]
- Fruzzetti, F.; Fidecicchi, T.; Guevara, M.M.M.; Simoncini, T. Estetrol: A New Choice for Contraception. J. Clin. Med. 2021, 10, 5625. [Google Scholar] [CrossRef]
- Watson, C.S.; Jeng, Y.; Kochukov, M.Y. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation. FASEB J. 2008, 22, 3328–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samavat, H.; Kurzer, M.S. Estrogen metabolism and breast cancer. Cancer Lett. 2015, 356, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.R. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Estradiol (Blood)—Health Encyclopedia—University of Rochester Medical Center. Available online: https://www.urmc.rochester.edu/encyclopedia/content.aspx?ContentTypeID=167&ContentID=estradiol (accessed on 11 December 2022).
- Handelsman, D.J.; Hirschberg, A.L.; Bermon, S. Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocr. Rev. 2018, 39, 803–829. [Google Scholar] [CrossRef] [Green Version]
- Testosterone Information | Mount Sinai—New York. Mount Sinai Health System. Available online: https://www.mountsinai.org/health-library/tests/testosterone (accessed on 11 December 2022).
- Brzozowska, M.; Lewiński, A. Changes of androgens levels in menopausal women. Menopausal Rev. 2020, 19, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef]
- Sarkar, M. Testosterone Levels in Women: Implications for Fatty Liver and Beyond. J. Women’s Health 2019, 28, 1015–1016. [Google Scholar] [CrossRef]
- Khan, M.S.; Kim, H.-S.; Kim, R.; Yoon, S.H.; Kim, S.G. Dysregulated Liver Metabolism and Polycystic Ovarian Syndrome. Int. J. Mol. Sci. 2023, 24, 7454. [Google Scholar] [CrossRef]
- Qu, X.; Donnelly, R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2020, 21, 8191. [Google Scholar] [CrossRef]
- Shen, M.; Shi, H. Sex Hormones and Their Receptors Regulate Liver Energy Homeostasis. Int. J. Endocrinol. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mody, A.; White, D.; Kanwal, F.; Garcia, J.M. Relevance of low testosterone to nonalcoholic fatty liver disease. Cardiovasc. Endocrinol. Metab. 2015, 4, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Sofer, Y.; Nevo, N.; Vechoropoulos, M.; Shefer, G.; Osher, E.; Landis, N.; Tordjman, K.; Hammond, G.L.; Stern, N. Human sex hormone-binding globulin does not provide metabolic protection against diet-induced obesity and dysglycemia in mice. Endocr. Connect. 2018, 7, 91–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, L.A.; Page, S.T.; Amory, J.K.; Anawalt, B.D.; Matsumoto, A.M. The association of obesity with sex hormone-binding globulin is stronger than the association with ageing—Implications for the interpretation of total testosterone measurements. Clin. Endocrinol. 2015, 83, 828–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagatell, C.J.; Bremner, W.J. Androgens in Men—Uses and Abuses. N. Engl. J. Med. 1996, 334, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L.; Ruokonen, A.; Kontturi, M.; Koskela, E.; Vihko, R. The Simultaneous Radioimmunoassay of Seven Steroids in Human Spermatic and Peripheral Venous Blood. J. Clin. Endocrinol. Metab. 1977, 45, 16–24. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Vermeulen, A. The Decline of Androgen Levels in Elderly Men and Its Clinical and Therapeutic Implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef]
- Supakar, P.C.; Roy, A.K. Role of Transcription Factors in the Age-Dependent Regulation of the Androgen Receptor Gene in Rat Liver. Neurosignals 1996, 5, 170–179. [Google Scholar] [CrossRef]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Baltimore Longitudinal Study of Aging. Longitudi-nal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A.; Winn, N.C.; Jurrissen, T.J.; Grunewald, Z.I.; Cunningham, R.P.; Woodford, M.L.; et al. Estrogens in Male Physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.B.; Wolf, B.; Peoples, C.; Fike, J.; Silver, K.; Laffoon, M.; Medsger, T.A., Jr.; Feghali-Bostwick, C. Estradiol levels are elevated in older men with diffuse cutaneous SSc and are associated with decreased survival. Arthritis Res. Ther. 2019, 21, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, A.; Kaufman, J.M.; Goemaere, S.; van Pottelberg, I. Estradiol in elderly men. Aging Male 2002, 5, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Kwakowsky, A.; Milne, M.R.; Waldvogel, H.J.; Faull, R.L. Effect of Estradiol on Neurotrophin Receptors in Basal Forebrain Cholinergic Neurons: Relevance for Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 2122. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Mayer, J.A.; Mazumdar, A.; Fertuck, K.; Kim, H.; Brown, M.; Brown, P.H. Estrogen Induces c-myc Gene Expression via an Upstream Enhancer Activated by the Estrogen Receptor and the AP-1 Transcription Factor. Mol. Endocrinol. 2011, 25, 1527–1538. [Google Scholar] [CrossRef] [Green Version]
- Bryzgalova, G.; Gao, H.; Ahren, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilssosn, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayaf, K.; Gabbia, D.; Russo, F.P.; De Martin, S. The Role of Sex in Acute and Chronic Liver Damage. Int. J. Mol. Sci. 2022, 23, 10654. [Google Scholar] [CrossRef]
- Beato, M. Gene regulation by steroid hormones. Cell 1989, 56, 335–344. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Yu, I.-C.; Wang, R.-S.; Chen, Y.-T.; Liu, N.-C.; Altuwaijri, S.; Hsu, C.-L.; Ma, W.-L.; Jokinen, J.; Sparks, J.D.; et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology 2008, 47, 1924–1935. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [Green Version]
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Hammes, S.R.; Levin, E.R. Extranuclear Steroid Receptors: Nature and Actions. Endocr. Rev. 2007, 28, 726–741. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Kumar, S.P.D.S.; Liu, X. G Protein-Coupled Estrogen Receptor in Energy Homeostasis and Obesity Pathogenesis. Prog. Mol. Biol. Transl. Sci. 1997, 138, 863–870. [Google Scholar]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Brown, T.R.; Yager, J.D. Mechanisms of hormone carcinogenesis: Evolution of views, role of mitochondria. Adv. Exp. Med. Biol. 2008, 630, 1–18. [Google Scholar]
- Klinge, C.M. Estrogenic control of mitochondrial function and biogenesis. J. Cell. Biochem. 2008, 105, 1342–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, D.-K.H.; Hartig, R.; Weinert, S.; Haybaeck, J.; Nass, N. G-Protein-Coupled Estrogen Receptor (GPER)-Specific Agonist G1 Induces ER Stress Leading to Cell Death in MCF-7 Cells. Biomolecules 2019, 9, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meoli, L.; Isensee, J.; Zazzu, V.; Nabzdyk, C.S.; Soewarto, D.; Witt, H.; Foryst-Ludwig, A.; Kintscher, U.; Noppinger, P.R. Sex- and age-dependent effects of Gpr30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene 2014, 540, 210–216. [Google Scholar] [CrossRef]
- Sharma, G.; Hu, C.; Brigman, J.L.; Zhu, G.; Hathaway, H.J.; Prossnitz, E.R. GPER Deficiency in Male Mice Results in Insulin Resistance, Dyslipidemia, and a Proinflammatory State. Endocrinology 2013, 154, 4136–4145. [Google Scholar] [CrossRef] [Green Version]
- Heinlein, C.A.; Chang, C. The Roles of Androgen Receptors and Androgen-Binding Proteins in Nongenomic Androgen Actions. Mol. Endocrinol. 2002, 16, 2181–2187. [Google Scholar] [CrossRef]
- Migliaccio, A.; Castoria, G.; Di Domenico, M.; De Falco, A.; Bilancio, A.; Lombardi, M.; Barone, M.V.; Ametrano, D.; Zannini, M.S.; Abbondanza, C.; et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000, 19, 5406–5417. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P.; Koc, E.; Sonpavde, G.; Singh, R.; Singh, K.K. Mitochondrial localization, import, and mitochondrial function of the androgen receptor. J. Biol. Chem. 2019, 294, 6621–6634. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Schwartzman, J.; Gibbs, A.; Lisac, R.; Kleinschmidt, R.; Wilmot, B.; Bottomly, D.; Coleman, I.; Nelson, P.; McWeeney, S.; et al. Androgen Receptor Promotes Ligand-Independent Prostate Cancer Progression through c-Myc Upregulation. PLoS ONE 2013, 8, e63563. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.-L.; Kyprianou, N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocrine-Relat. Cancer 2008, 15, 841–849. [Google Scholar] [CrossRef] [Green Version]
- Santen, R.J.; Brodie, H.; Simpson, E.R.; Siiteri, P.K.; Brodie, A. History of Aromatase: Saga of an Important Biological Mediator and Therapeutic Target. Endocr. Rev. 2009, 30, 343–375. [Google Scholar] [CrossRef]
- Narasaka, T.; Moriya, T.; Endoh, M.; Suzuki, T.; Shizawa, S.; Mizokami, Y.; Matsuoka, T.; Sasano, H. 17.BETA.-Hydroxysteroid Dehydrogenase Type 2 and Dehydroepiandrosterone Sulfotransferase in the Human Liver. Endocr. J. 2000, 47, 697–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kur, P.; Kolasa-Wołosiuk, A.; Misiakiewicz-Has, K.; Wiszniewska, B. Sex Hormone-Dependent Physiology and Diseases of Liver. Int. J. Environ. Res. Public Health 2020, 17, 2620. [Google Scholar] [CrossRef] [Green Version]
- Guillaume, M.; Riant, E.; Fabre, A.; Raymond-Letron, I.; Buscato, M.; Davezac, M.; Tramunt, B.; Montagner, A.; Smati, S.; Zahreddine, R.; et al. Selective Liver Estrogen Receptor α Modulation Prevents Steatosis, Diabetes, and Obesity Through the Anorectic Growth Differentiation Factor 15 Hepatokine in Mice. Hepatol. Commun. 2019, 3, 908–924. [Google Scholar] [CrossRef] [Green Version]
- Oliva, M.; Muñoz-Aguirre, M.; Kim-Hellmuth, S.; Wucher, V.; Gewirtz, A.D.H.; Cotter, D.J.; Parsana, P.; Kasela, S.; Balliu, B.; Viñuela, A.; et al. The impact of sex on gene expression across human tissues. Science 2020, 369, eaba3066. [Google Scholar] [CrossRef] [PubMed]
- Gerges, S.H.; El-Kadi, A.O. Sexual Dimorphism in the Expression of Cytochrome P450 Enzymes in Rat Heart, Liver, Kidney, Lung, Brain, and Small Intestine. Drug Metab. Dispos. 2023, 51, 81–94. [Google Scholar] [CrossRef]
- Meda, C.; Dolce, A.; Vegeto, E.; Maggi, A.; Della Torre, S. ERα-Dependent Regulation of Adropin Predicts Sex Differences in Liver Homeostasis during High-Fat Diet. Nutrients 2022, 14, 3262. [Google Scholar] [CrossRef]
- Trapani, L.; Pallottini, V. Age-Related Hypercholesterolemia and HMG-CoA Reductase Dysregulation: Sex Does Matter (A Gender Perspective). Curr. Gerontol. Geriatr. Res. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Trapani, L. Regulation and deregulation of cholesterol homeostasis: The liver as a metabolic “power station”. World J. Hepatol. 2012, 4, 184–190. [Google Scholar] [CrossRef]
- Lemieux, C.; Phaneuf, D.; Labrie, F.; Giguère, V.; Richard, D.; Deshaies, Y. Estrogen receptor α-mediated adiposity-lowering and hypocholesterolemic actions of the selective estrogen receptor modulator acolbifene. Int. J. Obes. 2005, 29, 1236–1244. [Google Scholar] [CrossRef] [Green Version]
- Pedram, A.; Razandi, M.; O’mahony, F.; Harvey, H.; Harvey, B.J.; Levin, E.R. Estrogen Reduces Lipid Content in the Liver Exclusively from Membrane Receptor Signaling. Sci. Signal. 2013, 6, ra36. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Kim, J.K.; O’Mahony, F.; Lee, E.Y.; Luderer, U.; Levin, E.R. Developmental Phenotype of a Membrane Only Estrogen Receptor α (MOER) Mouse. J. Biol. Chem. 2009, 284, 3488–3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-Q.; Terry, R.; Brown, T.R.; Russo, J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim. Biophys. Acta 2009, 1793, 1128–1143. [Google Scholar] [CrossRef] [Green Version]
- Im, S.-S.; Kim, J.-W.; Kim, T.-H.; Song, X.-L.; Kim, S.-Y.; Kim, H.-I.; Ahn, Y.-H. Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter. Exp. Mol. Med. 2005, 37, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, S.; Holmäng, A.; Björntorp, P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol. Scand. 1993, 149, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zirilli, L.; Rochira, V.; Diazzi, C.; Caffagni, G.; Carani, C. Human models of aromatase deficiency. J. Steroid Biochem. Mol. Biol. 2008, 109, 212–218. [Google Scholar] [CrossRef]
- Yamada, Y.; Ando, F.; Niino, N.; Ohta, S.; Shimokata, H. Association of polymorphisms of the estrogen receptor α gene with bone mineral density of the femoral neck in elderly Japanese women. J. Mol. Med. 2002, 80, 452–460. [Google Scholar] [CrossRef]
- Iavarone, M.; Lampertico, P.; Seletti, C.; Ronchi, G.; Del Ninno, E.; Colombo, M. The clinical and pathogenetic significance of estrogen receptor-? expression in chronic liver diseases and liver carcinoma. Cancer 2003, 98, 529–534. [Google Scholar] [CrossRef]
- Burra, P.; Bizzaro, D.; Gonta, A.; Shalaby, S.; Gambato, M.; Morelli, M.C.; Trapani, S.; Floreani, A.; Marra, F.; Brunetto, M.R.; et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021, 41, 1713–1733. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Meehan, A.G.; Shah, A. The Age Related Decrease in Testosterone is Significantly Exacerbated in Obese Men With the Metabolic Syndrome. What are the Implications for the Relatively High Incidence of Erectile Dysfunction Observed in These Men? J. Urol. 2006, 176, 1524–1527. [Google Scholar] [CrossRef]
- Völzke, H.; Aumann, N.; Krebs, A.; Nauck, M.; Steveling, A.; Lerch, M.M.; Rosskopf, D.; Wallaschofski, H. Hepatic steatosis is associated with low serum testosterone and high serum DHEAS levels in men. Int. J. Androl. 2010, 33, 45–53. [Google Scholar] [CrossRef]
- Kelly, D.M.; Nettleship, J.E.; Akhtar, S.; Muraleedharan, V.; Sellers, D.J.; Brooke, J.C.; McLaren, D.S.; Channer, K.S.; Jones, T.H. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci. 2014, 109, 95–103. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Xu, Q.; Yeh, S.; Wang, R.-S.; Sparks, J.D.; Chang, C. Insulin and Leptin Resistance with Hyperleptinemia in Mice Lacking Androgen Receptor. Diabetes 2005, 54, 1717–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, G.B.; Jing, T.; Heymsfield, S.B. Does insulin resistance, visceral adiposity, or a sex hormone alteration underlie the metabolic syndrome? Studies in women. Metabolism 2008, 57, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Tsatsoulis, A.; Zafeiriadou, E.; Katsiki, E.; Patsiaoura, K.; Zavos, C.; Anastasiadou, V.V.; Slavakis, A. Sex steroids and sex hormone-binding globulin in postmenopausal women with nonalcoholic fatty liver disease. Hormones 2013, 12, 405–416. [Google Scholar] [CrossRef]
- Klisic, A.; Kavaric, N.; Jovanovic, M.; Soldatovic, I.; Gligorovic-Barhanovic, N.; Kotur-Stevuljevic, J. Bioavailable testosterone is independently associated with Fatty Liver Index in postmenopausal women. Arch. Med. Sci. 2017, 5, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex Differences of Endogenous Sex Hormones and Risk of Type 2 Diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Muthusamy, T.; Murugesan, P.; Balasubramanian, K. Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective Akt phosphorylation in target tissues of adult male rat. Metabolism 2009, 58, 1581–1592. [Google Scholar] [CrossRef]
- Yeh, S.; Tsai, M.-Y.; Xu, Q.; Mu, X.-M.; Lardy, H.; Huang, K.-E.; Lin, H.; Yeh, S.-D.; Altuwaijri, S.; Zhou, X.; et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 13498–13503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Lee, S.O.; Wang, R.-S.; Yeh, S.; Chang, T.-M. Androgen Receptor (AR) Physiological Roles in Male and Female Reproductive Systems: Lessons Learned from AR-Knockout Mice Lacking AR in Selective Cells1. Biol. Reprod. 2013, 89, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, J.; Irwin, R.W.; Gallaher, T.K.; Brinton, R.D. Estradiol in vivo regulation of brain mitochondrial proteome. J. Neurosci. 2007, 27, 14069–14077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, J.; Fisher, B.M.; Jaap, A.J.; Stanley, A.; Paterson, K.; Sattar, N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: A randomized placebo-controlled trial. Clin. Endocrinol. 2006, 65, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Apostolov, R.; Gianatti, E.; Wong, D.; Kutaiba, N.; Gow, P.; Grossmann, M.; Sinclair, M. Testosterone therapy reduces hepatic steatosis in men with type 2 diabetes and low serum testosterone concentrations. World J. Hepatol. 2022, 14, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Dobs, A.S.; Nguyen, T.; Pace, C.; Roberts, C.P. Differential Effects of Oral Estrogen versus Oral Estrogen-Androgen Replacement Therapy on Body Composition in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2002, 87, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Hormone Therapy for Menopause: Types, Benefits & Risks. Cleveland Clinic. Available online: https://my.clevelandclinic.org/health/treatments/15245-hormone-therapy-for-menopause-symptoms (accessed on 11 December 2022).
- Ramasamy, R.; Osterberg, E.C.; Bernie, A.M. Risks of testosterone replacement therapy in men. Indian J. Urol. 2014, 30, 2–7. [Google Scholar] [CrossRef]
- Tassinari, R.; Tammaro, A.; Lori, G.; Tait, S.; Martinelli, A.; Cancemi, L.; Frassanito, P.; Maranghi, F. Risk Assessment of Transgender People: Development of Rodent Models Mimicking Gender-Affirming Hormone Therapies and Identification of Sex-Dimorphic Liver Genes as Novel Biomarkers of Sex Transition. Cells 2023, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Canbay, A.; Angulo, P.; Taniai, M.; Burgart, L.J.; Lindor, K.D.; Gores, G.J. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003, 125, 437–443. [Google Scholar] [CrossRef]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The Sterile Inflammatory Response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef] [Green Version]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose Tissue-Liver Cross Talk in the Control of Whole-Body Metabolism: Implications in Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Kakino, S.; Ohki, T.; Nakayama, H.; Yuan, X.; Otabe, S.; Hashinaga, T.; Wada, N.; Kurita, Y.; Tanaka, K.; Hara, K.; et al. Pivotal Role of TNF-α in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model. Horm. Metab. Res. 2017, 50, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Wandrer, F.; Liebig, S.; Marhenke, S.; Vogel, A.; John, K.; Manns, M.P.; Teufel, A.; Itzel, T.; Longerich, T.; Maier, O.; et al. TNF-Receptor-1 inhibition reduces liver steatosis, hepatocellular injury and fibrosis in NAFLD mice. Cell Death Dis. 2020, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.H.; Hirsova, P.; Tomita, K.; Bronk, S.F.; Werneburg, N.W.; Harrison, S.A.; Goodfellow, V.S.; Malhi, H.; Gores, G.J. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 2016, 63, 731–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-López, M.; Sánchez-Madrid, F.; Rodríguez-Frade, J.M.; Mellado, M.; Acevedo, A.; García, M.I.; Albar, J.P.; Martínez-A, C.; Marazuela, M. CXCR3 Chemokine Receptor Distribution in Normal and Inflamed Tissues: Expression on Activated Lymphocytes, Endothelial Cells, and Dendritic Cells. Lab. Investig. 2001, 81, 409–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, J.E.; McKenzie, T.J.; Lillegard, J.B.; Yu, Y.; Juskewitch, J.E.; Nedredal, G.I.; Brunn, G.J.; Yi, E.S.; Malhi, H.; Smyrk, T.C.; et al. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J. Surg. Res. 2012, 180, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu. Rev. Nutr. 1997, 17, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl–CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLoughlin, R.M.; Jenkins, B.J.; Grail, D.; Williams, A.S.; Fielding, C.A.; Parker, C.R.; Ernst, M.; Topley, N.; Jones, S.A. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc. Natl. Acad. Sci. USA 2005, 102, 9589–9594. [Google Scholar] [CrossRef] [Green Version]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Harbour, S.N.; DiToro, D.F.; Witte, S.J.; Zindl, C.L.; Gao, M.; Schoeb, T.R.; Jones, G.W.; Jones, S.A.; Hatton, R.D.; Weaver, C.T. T H 17 cells require ongoing classic IL-6 receptor signaling to retain transcriptional and functional identity. Sci. Immunol. 2020, 5, eaaw2262. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Schilling, A.-K.; Meertens, J.; Hering, I.; Weiss, J.; Jurowich, C.; Kudlich, T.; Hermanns, H.M.; Bantel, H.; Beyersdorf, N.; et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016, 196, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Sinton, M.C.; Girard, A.; Ogunsola, J.; Chandrasegaran, P.; Capewell, P.; Perona-Wright, G.; Quintana, J.F. Interleukin-17 drives sex-dependent weight loss and changes in feeding behaviour during Trypanosoma brucei infection. bioRxiv 2022. [Google Scholar] [CrossRef]
- Giles, D.A.; Moreno-Fernandez, M.E.; Stankiewicz, T.E.; Graspeuntner, S.; Cappelletti, M.; Wu, D.; Mukherjee, R.; Chan, C.C.; Lawson, M.J.; Klarquist, J.; et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 2017, 23, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Zúñiga, L.A.; Shen, W.-J.; Joyce-Shaikh, B.; Pyatnova, E.A.; Richards, A.G.; Thom, C.; Andrade, S.M.; Cua, D.J.; Kraemer, F.B.; Butcher, E.C. IL-17 Regulates Adipogenesis, Glucose Homeostasis, and Obesity. J. Immunol. 2010, 185, 6947–6959. [Google Scholar] [CrossRef] [Green Version]
- Harley, I.T.; Stankiewicz, T.E.; Giles, D.A.; Softic, S.; Flick, L.M.; Cappelletti, M.; Sheridan, R.; Xanthakos, S.A.; Steinbrecher, K.A.; Sartor, R.B.; et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 2013, 59, 1830–1839. [Google Scholar] [CrossRef]
- Moreno-Fernandez, M.E.; Giles, D.A.; Oates, J.R.; Chan, C.C.; Damen, M.S.; Doll, J.R.; Stankiewicz, T.E.; Chen, X.; Chetal, K.; Karns, R.; et al. PKM2-dependent metabolic skewing of hepatic Th17 cells regulates pathogenesis of non-alcoholic fatty liver disease. Cell Metab. 2021, 33, 1187–1204.e9. [Google Scholar] [CrossRef]
- González-Terán, B.; Matesanz, N.; Nikolic, I.; Verdugo, M.A.; Sreeramkumar, V.; Hernández-Cosido, L.; Sabio, G. p38γ and p38δ reprogram liver metabolism by modulating neutrophil infiltration. EMBO J. 2016, 35, 536–552. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleukin-17 Signaling in Inflammatory, Kupffer Cells, and Hepatic Stellate Cells Exacerbates Liver Fibrosis in Mice. Gastroenterology 2012, 143, 765–776.e3. [Google Scholar] [CrossRef] [Green Version]
- Nair, B.; Nath, L.R. Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J. Recept. Signal Transduct. 2020, 40, 195–200. [Google Scholar] [CrossRef]
- Meszaros, K.; Bojta, J.; Bautista, A.P.; Lang, C.; Spitzer, J.J. Glucose utilization by Kupffer cells, endothelial cells, and granulocytes in endotoxemic rat liver. Am. J. Physiol. Liver Physiol. 1991, 260, G7–G12. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Guan, D.; Wang, S.; Chai, L.Y.A.; Xu, S.; Lam, K.-P. Glycolysis and Oxidative Phosphorylation Play Critical Roles in Natural Killer Cell Receptor-Mediated Natural Killer Cell Functions. Front. Immunol. 2020, 11, 202. [Google Scholar] [CrossRef] [Green Version]
- Pearce, E.L.; Pearce, E.J. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Spolarics, Z.; Bautista, A.; Spitzer, J. Primed pentose cycle activity supports production and elimination of superoxide anion in Kupffer cells from rats treated with endotoxin in vivo. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1993, 1179, 134–140. [Google Scholar] [CrossRef]
- Luo, B.; Wang, J.; Liu, Z.; Shen, Z.; Shi, R.; Liu, Y.-Q.; Jiang, M.; Wu, Y.; Zhang, Z. Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution. Nat. Commun. 2016, 7, 12177. [Google Scholar] [CrossRef] [Green Version]
- El-Benna, J.; Dang, P.M.-C.; Gougerot-Pocidalo, M.-A. Priming of the neutrophil NADPH oxidase activation: Role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin. Immunopathol. 2008, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Xia, J.; Yao, W.; Huang, F. Enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases. FASEB J. 2019, 33, 11640–11654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesarwi, O.A.; Moya, E.A.; Zhen, X.; Gautane, M.; Zhao, H.; Giró, P.W.; Alshebli, M.; McCarley, K.E.; Breen, E.C.; Malhotra, A. Hepatocyte HIF-1 and Intermittent Hypoxia Independently Impact Liver Fibrosis in Murine Nonalcoholic Fatty Liver Disease. Am. J. Respir. Cell Mol. Biol. 2021, 65, 390–402. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Mridha, A.R.; Wree, A.; Robertson, A.A.; Yeh, M.M.; Johnson, C.D.; Van Rooyen, D.M.; Haczeyni, F.; Teoh, N.C.-H.; Savard, C.; Ioannou, G.N.; et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 2017, 66, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Billingham, L.K.; Stoolman, J.S.; Vasan, K.; Rodriguez, A.E.; Poor, T.A.; Szibor, M.; Jacobs, H.T.; Reczek, C.R.; Rashidi, A.; Zhang, P.; et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 2022, 23, 692–704. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Symptoms & Causes of NAFLD & NASH | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/symptoms-causes (accessed on 11 December 2022).
- Definition & Facts of NAFLD & NASH | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts (accessed on 11 December 2022).
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among a Largely Middle-Aged Population Utilizing Ultrasound and Liver Biopsy: A Prospective Study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease—An Evolving View. Clin. Liver Dis. 2017, 22, 11–21. [Google Scholar] [CrossRef]
- Spengler, E.K.; Loomba, R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin. Proc. 2015, 90, 1233–1246. [Google Scholar] [CrossRef] [Green Version]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T. Staging of liver fibrosis or cirrhosis: The role of hepatic venous pressure gradient measurement. World J. Hepatol. 2015, 7, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wong, V.W.-S.; Castellanos, M.; La Fuente, R.A.-D.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Sanz, M.A.-Q.; Conde-Martín, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e417. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- New Study shows 1 in 8 adults may have NASH—A more serious form of non-alcohol related fatty liver disease—British Liver Trust. Available online: https://britishlivertrust.org.uk/study-shows-1-in-8-may-have-nash/ (accessed on 11 December 2022).
- Noureddin, M.; Vipani, A.; Bresee, C.; Todo, T.; Kim, I.K.; Alkhouri, N.; Setiawan, V.; Tran, T.; Ayoub, W.S.; Lu, S.C.; et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications for Liver Transplant and Ethnic and Gender Variances. Am. J. Gastroenterol. 2018, 113, 1649–1659. [Google Scholar] [CrossRef]
- Hashimoto, E.; Tokushige, K. Prevalence, gender, ethnic variations, and prognosis of NASH. J. Gastroenterol. 2010, 46, 63–69. [Google Scholar] [CrossRef]
- Shao, G.; Liu, Y.; Lu, L.; Zhang, G.; Zhou, W.; Wu, T.; Wang, L.; Xu, H.; Ji, G. The Pathogenesis of HCC Driven by NASH and the Preventive and Therapeutic Effects of Natural Products. Front. Pharmacol. 2022, 13, 944088. [Google Scholar] [CrossRef]
- Dufour, J.-F.; Anstee, Q.M.; Bugianesi, E.; Harrison, S.; Loomba, R.; Paradis, V.; Tilg, H.; Wong, V.W.-S.; Zelber-Sagi, S. Current therapies and new developments in NASH. Gut 2022, 71, 2123–2134. [Google Scholar] [CrossRef]
- Soldatow, V.Y.; LeCluyse, E.L.; Griffith, L.G.; Rusyn, I. In vitro models for liver toxicity testing. Toxicol. Res. 2013, 2, 23–39. [Google Scholar] [CrossRef] [Green Version]
- LeCluyse, E.L. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur. J. Pharm. Sci. 2001, 13, 343–368. [Google Scholar] [CrossRef] [PubMed]
- LeCluyse, E.L.; Alexandre, E.; Hamilton, G.A.; Viollon-Abadie, C.; Coon, D.J.; Jolley, S.; Richert, L. Isolation and Culture of Primary Human Hepatocytes. Methods Mol. Biol. 2005, 290, 207–230. [Google Scholar] [CrossRef]
- Bhogal, R.H.; Hodson, J.; Bartlett, D.; Weston, C.J.; Curbishley, S.M.; Haughton, E.; Williams, K.T.; Reynolds, G.M.; Newsome, P.; Adams, D.; et al. Isolation of Primary Human Hepatocytes from Normal and Diseased Liver Tissue: A One Hundred Liver Experience. PLoS ONE 2011, 6, e18222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnier, D.; Li, R.; Delbos, F.; Fourrier, A.; Collet, C.; Guguen-Guillouzo, C.; Chesné, C.; Nguyen, T.H. Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Sci. Rep. 2018, 8, 8222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 2014, 160, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Gehart, H.; Artegiani, B.; Löpez-Iglesias, C.; Dekkers, F.; Basak, O.; Van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, D.; Artegiani, B.; Hu, H.; Lopes, S.C.d.S.; Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 2020, 16, 182–217. [Google Scholar] [CrossRef]
- Wesley, B.T.; Ross, A.D.B.; Muraro, D.; Miao, Z.; Saxton, S.; Tomaz, R.A.; Morell, C.M.; Ridley, K.; Zacharis, E.D.; Petrus-Reurer, S.; et al. Single-cell atlas of human liver development reveals pathways directing hepatic cell fates. Nature 2022, 24, 1487–1498. [Google Scholar] [CrossRef]
- He, Y.; Zhou, J.-W.; Xu, L.; Gong, M.-J.; He, T.-C.; Bi, Y. Comparison of proliferation and differentiation potential between mouse primary hepatocytes and embryonic hepatic progenitor cells in vitro. Int. J. Mol. Med. 2013, 32, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.-Y.; Bird, T.G.; Boulter, L.; Tsuchiya, A.; Cole, A.M.; Hay, T.; Guest, R.V.; Wojtacha, D.; Man, T.Y.; Mackinnon, A.; et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nature 2015, 17, 971–983. [Google Scholar] [CrossRef] [Green Version]
- Katsuda, T.; Kawamata, M.; Hagiwara, K.; Takahashi, R.-U.; Yamamoto, Y.; Camargo, F.D.; Ochiya, T. Conversion of Terminally Committed Hepatocytes to Culturable Bipotent Progenitor Cells with Regenerative Capacity. Cell Stem Cell 2016, 20, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuda, T.; Ochiya, T. Chemically Induced Liver Progenitors (CLiPs): A Novel Cell Source for Hepatocytes and Biliary Epithelial Cells: Methods and Protocols; Springer: New York, NY, USA, 2019; pp. 117–130. [Google Scholar]
- Yamaguchi, T.; Matsuzaki, J.; Katsuda, T.; Saito, Y.; Saito, H.; Ochiya, T. Generation of functional human hepatocytes in vitro: Current status and future prospects. Inflamm. Regen. 2019, 39, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.J.; Bandiera, L.; Menolascina, F.; Fallowfield, J.A. In vitro models for non-alcoholic fatty liver disease: Emerging platforms and their applications. iScience 2021, 25, 103549. [Google Scholar] [CrossRef]
- Soret, P.-A.; Magusto, J.; Housset, C.; Gautheron, J. In Vitro and In Vivo Models of Non-Alcoholic Fatty Liver Disease: A Critical Appraisal. J. Clin. Med. 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.L.; Takebe, T. Human liver model systems in a dish. Dev. Growth Differ. 2021, 63, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sinton, M.C.; Meseguer-Ripolles, J.; Lucendo-Villarin, B.; Wernig-Zorc, S.; Thomson, J.P.; Carter, R.N.; Lyall, M.J.; Walker, P.D.; Thakker, A.; Meehan, R.R.; et al. A human pluripotent stem cell model for the analysis of metabolic dysfunction in hepatic steatosis. iScience 2020, 24, 101931. [Google Scholar] [CrossRef]
- Sinton, M.C.; Meseguer-Ripolles, J.; Lucendo-Villarin, B.; Drake, A.J.; Hay, D.C. Modeling human hepatic steatosis in pluripotent stem cell-derived hepatocytes. STAR Protoc. 2021, 2, 100493. [Google Scholar] [CrossRef]
- Parafati, M.; Kirby, R.J.; Khorasanizadeh, S.; Rastinejad, F.; Malany, S. A nonalcoholic fatty liver disease model in human induced pluripotent stem cell-derived hepatocytes, created by endoplasmic reticulum stress-induced steatosis. Dis. Model. Mech. 2018, 11, dmm033530. [Google Scholar] [CrossRef] [Green Version]
- Tilson, S.G.; Morell, C.M.; Lenaerts, A.; Park, S.B.; Hu, Z.; Jenkins, B.; Koulman, A.; Liang, T.J.; Vallier, L. Modeling PNPLA3-Associated NAFLD Using Human-Induced Pluripotent Stem Cells. Hepatology 2021, 74, 2998–3017. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, R.; Togo, S.; Kimura, M.; Shinozawa, T.; Koido, M.; Koike, H.; Thompson, W.; Karns, R.A.; Mayhew, C.N.; McGrath, P.S.; et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019, 30, 374–384.e6. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasarinaite, A.; Sinton, M.; Saunders, P.T.K.; Hay, D.C. The Influence of Sex Hormones in Liver Function and Disease. Cells 2023, 12, 1604. https://doi.org/10.3390/cells12121604
Kasarinaite A, Sinton M, Saunders PTK, Hay DC. The Influence of Sex Hormones in Liver Function and Disease. Cells. 2023; 12(12):1604. https://doi.org/10.3390/cells12121604
Chicago/Turabian StyleKasarinaite, Alvile, Matthew Sinton, Philippa T. K. Saunders, and David C. Hay. 2023. "The Influence of Sex Hormones in Liver Function and Disease" Cells 12, no. 12: 1604. https://doi.org/10.3390/cells12121604