Pd-Bi-Based Catalysts for Selective Oxidation of Glucose into Gluconic Acid: The Role of Local Environment of Nanoparticles in Dependence of Their Composition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metal Content and Surface Characterization Using Electron Microscopy
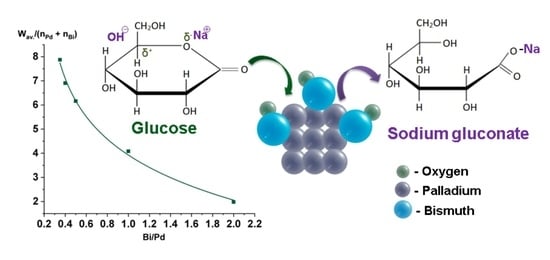
2.2. Catalytic Experiments
2.3. EXAFS and XANES Methods of Sample Characterization
3. Materials and Methods
3.1. Catalyst Preparation
3.2. X-ray Fluorescence Analysis (XRF)
3.3. Scanning Electron Microscopy and Energy-Dispersive Spectroscopy (SEM-EDS)
3.4. Transmission Electron Microscopy and Energy-Dispersive Spectrometry
3.5. Research of Catalytic Properties
3.6. EXAFS and XANES Methods of Sample Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, L.; Zheng, L.; Wei, H.; Zheng, S.; Zhu, Z.; Chen, J.; Yang, D. Palladium/bismuth nanowires with rough surface for stable hydrogen sensing at low temperatures. Appl. Nano Mater. 2019, 2, 1178–1184. [Google Scholar] [CrossRef]
- Kansara, S.; Gupta, S.K.; Sonvane, Y.; Gajjar, P.N. Ultrathin Pd and Pt nanowires for potential applications as hydrogen economy. Mater. Today Commun. 2021, 26, 101761. [Google Scholar] [CrossRef]
- Chu, M.; Huang, J.; Gong, J.; Qu, Y.; Chen, G.; Yang, H.; Wang, X.; Zhong, Q.; Deng, C.; Cao, M.; et al. Synergistic combination of Pd nanosheets and porous Bi(OH)3 boosts activity and durability for ethanol oxidation reaction. Nano Res. 2022, 15, 3920–3926. [Google Scholar] [CrossRef]
- Casella, I.G.; Contursi, M. Characterization of bismuth adatom-modified palladium electrodes: The electrocatalytic oxidation of aliphatic aldehydes in alkaline solutions. Electrochim. Acta 2006, 52, 649–657. [Google Scholar] [CrossRef]
- Witońska, I.A.; Walock, M.J.; Dziugan, P.; Karski, S.; Stanishevsky, A.V. The structure of Pd–M supported catalysts used in the hydrogen transfer reactions (M = In, Bi and Te). Appl. Surf. Sci. 2013, 273, 330–342. [Google Scholar] [CrossRef]
- Qin, X.; Li, H.; Xie, S.; Li, K.; Jiang, T.; Ma, X.Y.; Jiang, K.; Zhang, Q.; Terasaki, O.; Wu, Z. Mechanistic analysis-guided Pd-based catalysts for efficient hydrogen production from formic acid dehydrogenation. ACS Catal. 2020, 10, 3921–3932. [Google Scholar] [CrossRef]
- Shen, T.; Chen, S.; Zeng, R.; Gong, M.; Zhao, T.; Lu, Y.; Liu, X.; Xiao, D.; Yang, Y.; Hu, J.; et al. Tailoring the antipoisoning performance of Pd for formic acid electrooxidation via an ordered PdBi intermetallic. ACS Catal. 2020, 10, 9977–9985. [Google Scholar] [CrossRef]
- Witońska, I.; Królak, A.; Karski, S. Bi modified Pd/support (SiO2, Al2O3) catalysts for hydrodechlorination of 2, 4-dichlorophenol. J. Mol. Catal. A Chem. 2010, 331, 21–28. [Google Scholar] [CrossRef]
- Belkacemi, K.; Hamoudi, S. Chemocatalytic oxidation of lactose to lactobionic acid over Pd-Bi/SBA-15: Reaction kinetics and modeling. Ind. Eng. Chem. Res. 2010, 49, 6878–6889. [Google Scholar] [CrossRef]
- Karski, S. Catalytic oxidation of lactose over Pd-Bi/SiO2 systems. Przem. Chem. 2006, 85, 201–204. [Google Scholar]
- Wenkin, M.; Ruiz, P.; Delmon, B.; Devillers, M. The role of bismuth as promoter in Pd–Bi catalysts for the selective oxidation of glucose to gluconate. J. Mol. Catal. A Chem. 2002, 180, 141–159. [Google Scholar] [CrossRef]
- Karski, S.; Paryjczak, T.; Witonñska, I. Selective oxidation of glucose to gluconic acid over bimetallic Pd–Me catalysts (Me = Bi, Tl, Sn, Co). Kinet. Catal. 2003, 44, 618–622. [Google Scholar] [CrossRef]
- Sandu, M.P.; Sidelnikov, V.S.; Geraskin, A.A.; Chernyavskii, A.V.; Kurzina, I.A. Influence of the method of preparation of the Pd-Bi/Al2O3 catalyst on catalytic properties in the reaction of liquid-phase oxidation of glucose into gluconic acid. Catalysts 2020, 10, 271. [Google Scholar] [CrossRef]
- Singh, S.B.; Tandon, P.K. Catalysis: A brief review on nano-catalyst. J. Energy Chem. Eng. 2014, 2, 106–115. [Google Scholar]
- Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface coordination chemistry of metal nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131. [Google Scholar] [CrossRef]
- Mitchell, S.; Qin, R.; Zheng, N.; Pérez-Ramírez, J. Nanoscale engineering of catalytic materials for sustainable technologies. Nat. Nanotechnol. 2021, 16, 129–139. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Bimetallic nanocrystals: Liquid-phase synthesis and catalytic applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef]
- van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Pérez-Lorenzo, M. Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Liu, D.; He, Q.; Ding, S.; Song, L. Structural regulation and support coupling effect of single-atom catalysts for heterogeneous catalysis. Adv. Energy Mater. 2020, 10, 2001482. [Google Scholar] [CrossRef]
- Liu, J. Advanced electron microscopy of metal–support interactions in supported metal catalysts. ChemCatChem 2011, 3, 934–948. [Google Scholar] [CrossRef]
- Gallezot, P. Catalytic routes from renewables to fine chemicals. Catal. Today 2007, 121, 76–91. [Google Scholar] [CrossRef]
- Sarkar, S.; Ramarao, S.D.; Das, T.; Das, R.; Vinod, C.P.; Chakraborty, S.; Peter, S.C. Unveiling the roles of lattice strain and descriptor species on Pt-like oxygen reduction activity in Pd–Bi catalysts. ACS Catal. 2021, 11, 800–808. [Google Scholar] [CrossRef]
- Timoshenko, J.; Roldan Cuenya, B. In Situ/operando electrocatalyst characterization by X-ray absorption spectroscopy. Chem. Rev. 2020, 121, 882–961. [Google Scholar] [CrossRef]
- Finzel, J.; Gutierrezet, K.M.S.; Hoffman, A.S.; Resasco, J.; Christopher, P.; Bare, S.R. Limits of Detection for EXAFS Characterization of Heterogeneous Single-Atom Catalysts. ACS Catal. 2023, 13, 6462–6473. [Google Scholar] [CrossRef]
- Mondal, S.; Ballav, T.; Biswas, K.; Ghosh, S.; Ganesh, V. Exploiting the versatility of palladium catalysis: A modern toolbox for cascade reactions. Eur. J. Org. Chem. 2021, 2021, 4566–4602. [Google Scholar] [CrossRef]
- Wenkin, M.; Renard, C.; Ruiz, P.; Delmon, B.; Devillers, M. On the role of bismuth-based alloys in carbon-supported bimetallic Bi-Pd catalysts for the selective oxidation of gluconic acid. Stud. Surf. Sci. Catal. 1997, 108, 391–398. [Google Scholar] [CrossRef]
- Witkowska, A.; Rybicki, J.; Di Cicco, A. Structure of partially reduced bismuth–silicate glasses: EXAFS and MD study. J. Alloys Compd. 2005, 401, 135–144. [Google Scholar] [CrossRef]
- Kremneva, A.M.; Fedorov, A.V.; Bulavchenko, O.A.; Knyazev, Y.V.; Saraev, A.A.; Yakovlev, V.A.; Kaichev, V.V. Effect of Calcination Temperature on Activity of Fe2O3–Al2O3 Nanocomposite Catalysts in CO Oxidation. Catal. Lett. 2020, 150, 3377–3385. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Tellgren, R.; Rundlo, H.; Orlov, V.G. Structural studies of α-Bi2O3 by neutron powder diffraction. Powder Diffr. 2001, 16, 227–230. [Google Scholar] [CrossRef]
- Bayliss, P. Revised unit-cell dimensions, space group, and chemical formula of some metallic minerals. Can. Mineral. 1990, 28, 751–755. [Google Scholar]
- Escola, J.M.; Serrano, D.P.; Aguado, J.; Briones, L. Hydroreforming of the LDPE thermal cracking oil over hierarchical Ni/beta catalysts with different Ni particle size distributions. Ind. Eng. Chem. Res. 2015, 54, 6660–6668. [Google Scholar] [CrossRef]
- Romero, M.D.; Calles, J.A.; Rodríguez, A. Influence of the Preparation Method and Metal Precursor Compound on the Bifunctional Ni/HZSM5 Catalysts. Ind. Eng. Chem. Res. 1997, 36, 3533. [Google Scholar] [CrossRef]
- Karski, S. Activity and selectivity of Pd–Bi/SiO2 catalysts in the light of mutual interaction between Pd and B. J. Mol. Catal. A Chem. 2006, 253, 147–154. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural Materials Science end-station at the Kurchatov Synchrotron Radiation Source: Recent instrumentation upgrades and experimental results. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Rehr, J.J.; Mustre de Leon, J.; Zabinsky, S.I.; Albers, R.C. Theoretical X-ray absorption fine structure standards. J. Am. Chem. Soc. 1991, 113, 5135–5140. [Google Scholar] [CrossRef]






| Sample/Al2O3 | Pd Content, wt. % | Bi Content, wt. % |
|---|---|---|
| 0.35Bi:Pd | 3.5 ± 0.3 | 2.4 ± 0.2 |
| 0.4Bi:Pd | 2.8 ± 0.3 | 2.3 ± 0.2 |
| 0.5Bi:Pd | 2.5 ± 0.2 | 2.3 ± 0.2 |
| 1Bi:Pd | 2.3 ± 0.2 | 2.4 ± 0.2 |
| 2Bi:Pd | 1.1 ± 0.1 | 3.9 ± 0.3 |
| Sample | Pd Content, at. % | Bi Content, at. % | Al Content, at. % | O Content, at. % | Ratio Bi/Pd |
|---|---|---|---|---|---|
| 0.35Bi:Pd | 1.35 ± 0.13 | 0.44 ± 0.04 | 46.33 ± 4.63 | 51.88 ± 5.19 | 0.33 |
| 0.4Bi:Pd | 0.36 ± 0.04 | 0.14 ± 0.01 | 37.98 ± 3.80 | 61.51 ± 6.15 | 0.39 |
| 0.5Bi:Pd | 1.37 ± 0.14 | 0.70 ± 0.07 | 41.73 ± 4.17 | 56.20 ± 5.62 | 0.51 |
| 1Bi:Pd | 0.56 ± 0.05 | 0.56 ± 0.06 | 37.66 ± 3.77 | 61.21 ± 6.12 | 1.00 |
| 2Bi:Pd | 0.47 ± 0.05 | 0.93 ± 0.09 | 42.34 ± 4.23 | 56.26 ± 5.63 | 1.97 |
| Sample/Al2O3 | Conversion XGlu, % | Yield YGluNa,% | Selectivity SGluNa, % | W0·105, mol/(l·s) | Wav·105, mol/(l·s) |
|---|---|---|---|---|---|
| Pd | 29.1 ± 1.1 | 27.1 ± 1.0 | 93 | 5.0 ± 0.2 | 1.9 ± 0.1 |
| 0.35Bi:Pd | 56.6 ± 1.2 | 56.6 ± 1.2 | 99 | 8.6 ± 0.4 | 3.8 ± 0.2 |
| 0.4Bi:Pd | 52.5 ± 2.1 | 51.8 ± 2.0 | 99 | 7.8 ± 0.4 | 3.5 ± 0.2 |
| 0.5Bi:Pd | 47.5 ± 1.4 | 47.4 ± 1.4 | 99 | 8.0 ± 0.4 | 3.2 ± 0.2 |
| 1Bi:Pd | 42.1 ± 1.3 | 42.1 ± 1.3 | >99 | 5.2 ± 0.2 | 2.8 ± 0.2 |
| 2Bi:Pd | 27.8 ± 0.9 | 27.8 ± 0.9 | >99 | 4.9 ± 0.2 | 1.8 ± 0.1 |
| No | Sample/Al2O3 | Bi, at. % | Bi2O3, at. % |
|---|---|---|---|
| 1 | 0.35Bi:Pd | 30.0 ± 1.5 | 70.0 ± 3.5 |
| 2 | 0.4Bi:Pd | 30.0 ± 1.5 | 70.0 ± 3.5 |
| 3 | 0.5Bi:Pd | 30.0 ± 1.5 | 70.0 ± 3.5 |
| 4 | 1Bi:Pd | 30.0 ± 1.5 | 70.0 ± 3.5 |
| 5 | 2Bi:Pd | 30.0 ± 1.5 | 70.0 ± 3.5 |
| Sample/Al2O3 | NBi-O | RBi-O, Å | σ2, Å2 | NBi-Pd | RBi-Pd, Å | σ2, Å2 | Rf, % |
|---|---|---|---|---|---|---|---|
| 0.35Bi:Pd | 2.8 | 2.14 | 0.008 | 3.7 | 2.79 | 0.011 | 0.8 |
| 0.4Bi:Pd | 3.7 | 2.15 | 0.008 | 2.4 | 2.78 | 0.011 | 0.8 |
| 0.5Bi:Pd | 3.6 | 2.14 | 0.008 | 2.5 | 2.77 | 0.011 | 1.3 |
| 1Bi:Pd | 3.7 | 2.13 | 0.008 | 1.7 | 2.76 | 0.011 | 0.8 |
| 2Bi:Pd | 3.7 | 2.14 | 0.008 | 1.2 | 2.77 | 0.011 | 0.8 |
| Bi2O3 | 4.0 | 2.16 | – | – | – | – | – |
| PdBi | – | – | – | 6 | 2.83 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbakova-Sandu, M.P.; Saraev, A.A.; Knyazev, A.S.; Kurzina, I.A. Pd-Bi-Based Catalysts for Selective Oxidation of Glucose into Gluconic Acid: The Role of Local Environment of Nanoparticles in Dependence of Their Composition. Catalysts 2024, 14, 66. https://doi.org/10.3390/catal14010066
Shcherbakova-Sandu MP, Saraev AA, Knyazev AS, Kurzina IA. Pd-Bi-Based Catalysts for Selective Oxidation of Glucose into Gluconic Acid: The Role of Local Environment of Nanoparticles in Dependence of Their Composition. Catalysts. 2024; 14(1):66. https://doi.org/10.3390/catal14010066
Chicago/Turabian StyleShcherbakova-Sandu, Mariya P., Andrey A. Saraev, Alexey S. Knyazev, and Irina A. Kurzina. 2024. "Pd-Bi-Based Catalysts for Selective Oxidation of Glucose into Gluconic Acid: The Role of Local Environment of Nanoparticles in Dependence of Their Composition" Catalysts 14, no. 1: 66. https://doi.org/10.3390/catal14010066